
How would you make a Bohr diagram for $NaCl$?
Answer
558.9k+ views
Hint: The basic concept of chemistry which tells that the Bohr’s atomic model consists of a central nucleus which is formed by the protons and neutrons together and then the circular orbits in which the electrons revolve. Draw the diagram for individual atoms and then combine to get the whole compound.
Complete step – by – step answer:
The concept of chemistry which deals with the topic called some basic concept of chemistry tells us about the various models for atoms given by the scientists among which the Bohr’ model is the one.
Now we shall see how to draw the Bohr diagram for sodium chloride.
- Bohr’s atomic model consists of a central nucleus which is formed by the protons and neutrons together and then the circular orbits in which the electrons revolve.
- To draw the Bohr’s atomic model for the compound let us first draw for the individual atoms.
- Sodium is having total of 11 protons and 11 electrons and the number of neutrons is given by the difference of mass number and the number of protons and that is $23-11=12$
Thus, the Bohr’s model for sodium is as shown below,
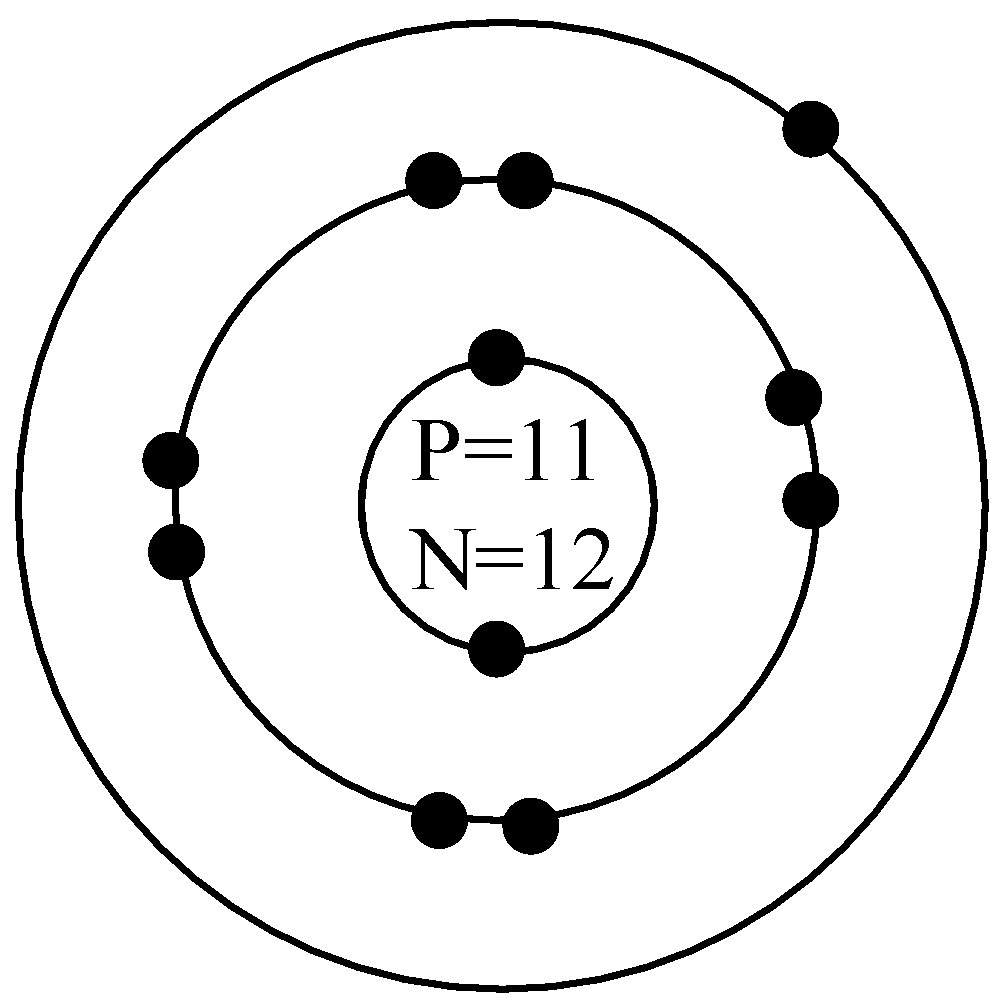
Similarly for the chlorine atom with atomic number 17 and mass number 35 has total 17 protons and 17 electrons with the neutrons having$35-17=18$in number. The Bohr’s model for this atom will be,
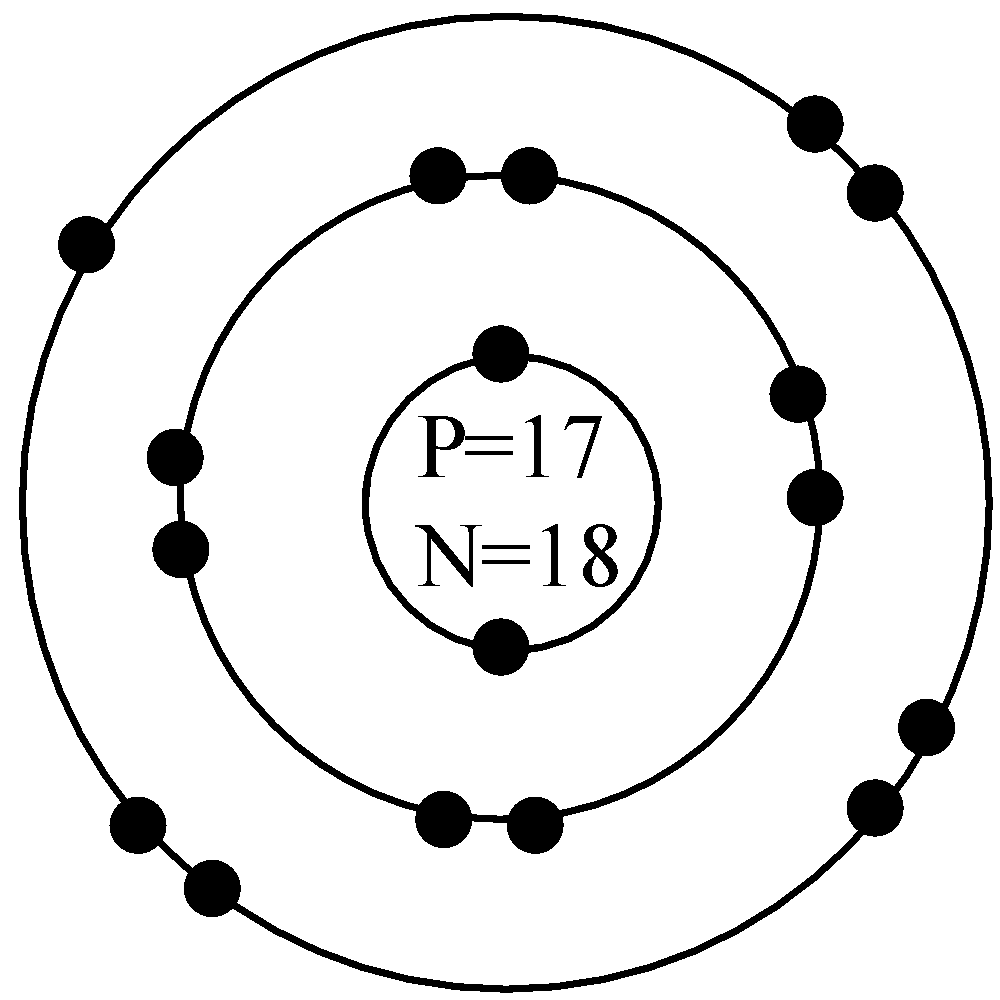
Now, since the compound sodium chloride is ionic in nature, the sodium ion donates its one electron from the third valence shell to chlorine and gets a positive charge whereas chlorine accepts the electron from sodium to become a negatively charged species and this can be shown in the combined diagram of Bohr’s model as,
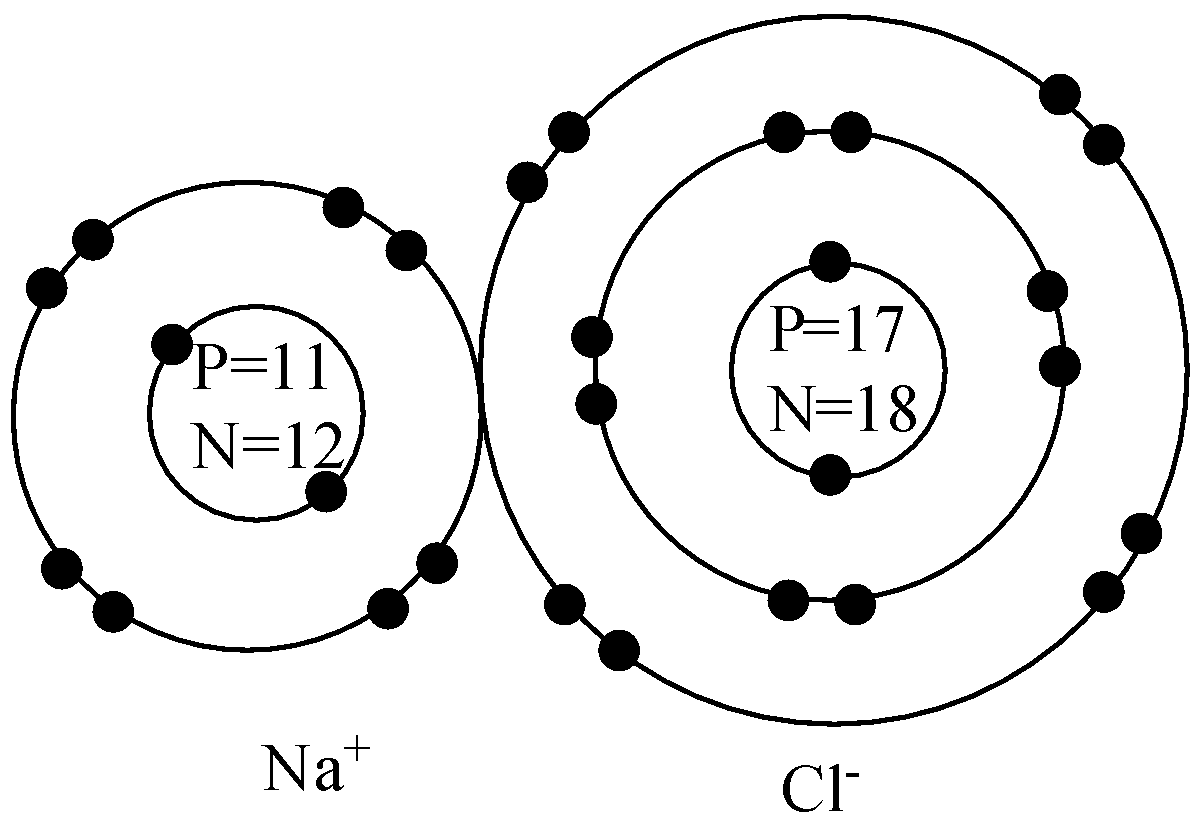
Note: Note that the atomic radius sodium decreases when it forms an ionic bond with chlorine and this is because the sodium donates the electron which is present in its outermost shell and the remaining shell is only 2 after donation.
Complete step – by – step answer:
The concept of chemistry which deals with the topic called some basic concept of chemistry tells us about the various models for atoms given by the scientists among which the Bohr’ model is the one.
Now we shall see how to draw the Bohr diagram for sodium chloride.
- Bohr’s atomic model consists of a central nucleus which is formed by the protons and neutrons together and then the circular orbits in which the electrons revolve.
- To draw the Bohr’s atomic model for the compound let us first draw for the individual atoms.
- Sodium is having total of 11 protons and 11 electrons and the number of neutrons is given by the difference of mass number and the number of protons and that is $23-11=12$
Thus, the Bohr’s model for sodium is as shown below,

Similarly for the chlorine atom with atomic number 17 and mass number 35 has total 17 protons and 17 electrons with the neutrons having$35-17=18$in number. The Bohr’s model for this atom will be,

Now, since the compound sodium chloride is ionic in nature, the sodium ion donates its one electron from the third valence shell to chlorine and gets a positive charge whereas chlorine accepts the electron from sodium to become a negatively charged species and this can be shown in the combined diagram of Bohr’s model as,

Note: Note that the atomic radius sodium decreases when it forms an ionic bond with chlorine and this is because the sodium donates the electron which is present in its outermost shell and the remaining shell is only 2 after donation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




