
What is the magnetic moment of ${[Fe{F_6}]^{3 - }}$ ?
(A). $5.91$
(B). $5.49$
(C). $2.32$
(D). $4$
Answer
506.1k+ views
Hint: Magnetic moment is defined as the magnetic strength and orientation of a magnetic or other object that produces a magnetic field. The direction of the magnetic moment points from the south to north pole of the magnet ( inside the magnet). The magnetic field of a magnetic dipole is proportional to its magnetic dipole. The SI unit is dipole.
Complete answer:
Given that, ${[Fe{F_6}]^{3 - }}$ we have to calculate the magnetic moment of $F{e^{3 + }}$ . the electronic configuration $[Ar]3{d^6}4{s^2}$
Here $Fe$ is given as $F{e^{3 + }}$ .
$F{e^{3 + }}$ is formed when $Fe$ donates three electrons. Thus the configuration of $F{e^{3 + }}$ is $[Ar]3{d^5}$ .
To calculate the magnetic moment of an ion the formula used is $\sqrt {n(n + 2)} $ , where n is the number of unpaired electrons.
The unpaired electrons in $F{e^{3 + }}$ is $5$ . As shown below:-
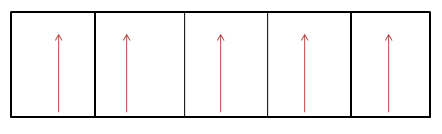
Substituting the value of unpaired electrons in the formula, we get
$\sqrt {5(5 + 2)} $$ \Rightarrow \sqrt {5(7)} $$ \Rightarrow \sqrt {35} \Rightarrow 5.91$ BM
Additional information: -
Iron (III) is used as follows: - (i) used as pigment, (ii) cosmetic, (iii) tattoo ink, (iv) used as a phosphate remover from home aquaria. Found in oxyhemoglobin, ferredoxin, cytochromes, etc.
Note:
The magnetic dipole moment is a vector field whose direction is perpendicular to the applied field and determined by the right hand thumb rule. In this rule the grip of the hand shows the direction of flow of current and the thumbs show the direction of the magnetic field. The magnetic moment is produced by two methods: motion electric charge and spin angular momentum.
Complete answer:
Given that, ${[Fe{F_6}]^{3 - }}$ we have to calculate the magnetic moment of $F{e^{3 + }}$ . the electronic configuration $[Ar]3{d^6}4{s^2}$
Here $Fe$ is given as $F{e^{3 + }}$ .
$F{e^{3 + }}$ is formed when $Fe$ donates three electrons. Thus the configuration of $F{e^{3 + }}$ is $[Ar]3{d^5}$ .
To calculate the magnetic moment of an ion the formula used is $\sqrt {n(n + 2)} $ , where n is the number of unpaired electrons.
The unpaired electrons in $F{e^{3 + }}$ is $5$ . As shown below:-

Substituting the value of unpaired electrons in the formula, we get
$\sqrt {5(5 + 2)} $$ \Rightarrow \sqrt {5(7)} $$ \Rightarrow \sqrt {35} \Rightarrow 5.91$ BM
Additional information: -
Iron (III) is used as follows: - (i) used as pigment, (ii) cosmetic, (iii) tattoo ink, (iv) used as a phosphate remover from home aquaria. Found in oxyhemoglobin, ferredoxin, cytochromes, etc.
Note:
The magnetic dipole moment is a vector field whose direction is perpendicular to the applied field and determined by the right hand thumb rule. In this rule the grip of the hand shows the direction of flow of current and the thumbs show the direction of the magnetic field. The magnetic moment is produced by two methods: motion electric charge and spin angular momentum.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




