
Loss of electrons is called:
(A) Reduction
(B) Oxidation
(C) Both (A) and (B)
(D) None
Answer
595.8k+ views
Hint: When an atom’s oxidation number increases, the process could be called oxidation process and when an atom’s oxidation number reduces, the process can be called reduction process.
Complete step by step answer:
To answer this question, we should know about oxidation, reduction and redox reaction. So, first we should know about these and then we will answer the question.
-In very simple terms that we can understand oxidation and reduction is the addition or removal of oxygen to a compound. Now, we will know about oxidation and reduction with respect to oxygen transfer. We should know that oxidation is the gain of oxygen and reduction is the loss of oxygen. And we should know that as both reduction and oxidation are occurring simultaneously, this is known as a redox reaction. Now, we will understand oxidation and reduction with respect to hydrogen transfer. We should note that oxidation is the loss of hydrogen and reduction is the gain of hydrogen.
We can also understand oxidation and reduction by using the concept of electron transfer. We should note that oxidation is loss of electrons whereas reduction is gain of electrons.
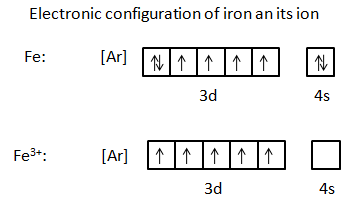
For example: In the above figure, when iron undergoes oxidation, it is transformed because it has lost electrons. Once iron has been oxidized, it carries a charge. Because it lost three electrons, it now has a positive charge of three. This positive three charge is represented by the number three and a positive sign (3+) written as a superscript to the right of the Iron (Fe) symbol.
So, from this we can say that our correct answer is option B. We came to know that addition of oxygen, or removal of hydrogen or loss of electron is oxidation.
So, the correct answer is “Option B”.
Note: We should note that reduction is the process of gaining one or more electrons. In an oxidation-reduction, or redox, reaction, one atom or compound will steal electrons from another atom or compound. A classic example of a redox reaction is rusting. When rusting happens, oxygen steals electrons from iron. Oxygen gets reduced while iron gets oxidized. The result is a compound called iron oxide, or rust.
Complete step by step answer:
To answer this question, we should know about oxidation, reduction and redox reaction. So, first we should know about these and then we will answer the question.
-In very simple terms that we can understand oxidation and reduction is the addition or removal of oxygen to a compound. Now, we will know about oxidation and reduction with respect to oxygen transfer. We should know that oxidation is the gain of oxygen and reduction is the loss of oxygen. And we should know that as both reduction and oxidation are occurring simultaneously, this is known as a redox reaction. Now, we will understand oxidation and reduction with respect to hydrogen transfer. We should note that oxidation is the loss of hydrogen and reduction is the gain of hydrogen.
We can also understand oxidation and reduction by using the concept of electron transfer. We should note that oxidation is loss of electrons whereas reduction is gain of electrons.

For example: In the above figure, when iron undergoes oxidation, it is transformed because it has lost electrons. Once iron has been oxidized, it carries a charge. Because it lost three electrons, it now has a positive charge of three. This positive three charge is represented by the number three and a positive sign (3+) written as a superscript to the right of the Iron (Fe) symbol.
So, from this we can say that our correct answer is option B. We came to know that addition of oxygen, or removal of hydrogen or loss of electron is oxidation.
So, the correct answer is “Option B”.
Note: We should note that reduction is the process of gaining one or more electrons. In an oxidation-reduction, or redox, reaction, one atom or compound will steal electrons from another atom or compound. A classic example of a redox reaction is rusting. When rusting happens, oxygen steals electrons from iron. Oxygen gets reduced while iron gets oxidized. The result is a compound called iron oxide, or rust.
Recently Updated Pages
Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Computer Science: Engaging Questions & Answers for Success

Class 10 Question and Answer - Your Ultimate Solutions Guide

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Trending doubts
A boat goes 24 km upstream and 28 km downstream in class 10 maths CBSE

State and explain Ohms law class 10 physics CBSE

Distinguish between soap and detergent class 10 chemistry CBSE

a Why did Mendel choose pea plants for his experiments class 10 biology CBSE

What is a "free hit" awarded for in limited-overs cricket?

Draw the diagram of the sectional view of the human class 10 biology CBSE




