
How many lone pairs are on the central atom in ${\text{XeO}}{{\text{F}}_{\text{2}}}$
?
Answer
555k+ views
Hint: We know that lone pair is a pair of valence electrons that is not used in formation of covalent bonds. And this electron pair is sometimes known as a nonbonding pair or unshared pair. Here, to know the number of lone pairs on the central atom, first we have to know the valence electron of Xe.
Complete step by step answer:
The given compound is a compound of xenon, that is, ${\text{XeO}}{{\text{F}}_{\text{2}}}$. Here, the xenon is bonded to one oxygen atom and two fluorine atoms.
We know that xenon is a noble gas that belongs to the group 18 of the periodic table. Its atomic number is 54. The electronic configuration of xenon is $\left[ {{\text{Kr}}} \right]4{d^{10}}5{s^2}5{p^6}$. So, the number of valence electrons of xenon is 8.
Now, we discuss the covalent bonding in ${\text{XeO}}{{\text{F}}_{\text{2}}}$. Out of 8 valence electrons, two are shared with the oxygen atom to form double covalent bond and one electron is shared with two fluorine atoms each to form two Xe-F single covalent bonds. So, four electrons remain unshared on the xenon atom.
Let’s understand with the help of the diagram.
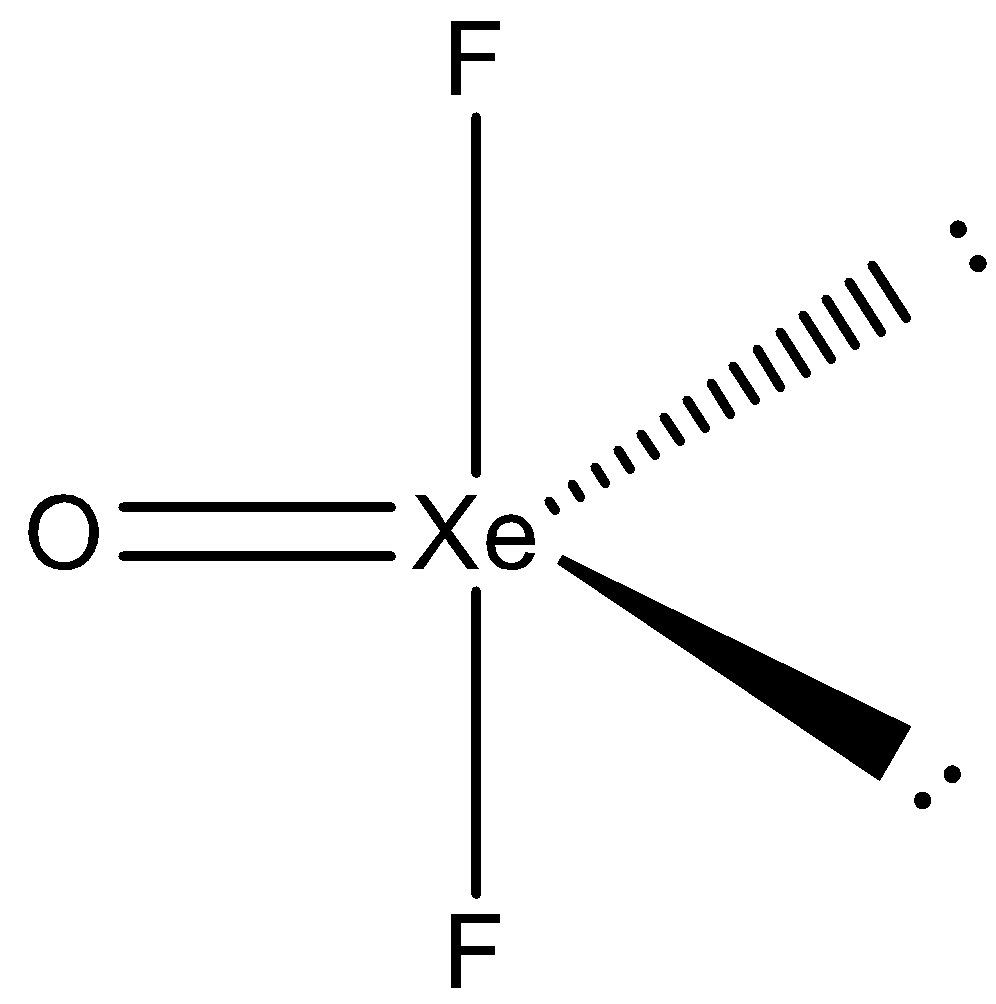
So, the number of lone pairs in ${\text{XeO}}{{\text{F}}_{\text{2}}}$ is 2.
Note: It is to be remembered that all elements belong to group 18 are termed as noble gas. These elements have a complete octet of valence shell, so they are inert in nature. The best characterized compounds are formed due to the reaction of xenon with oxygen and fluorine under certain conditions. Some example of xenon compounds are ${\text{XeO}}{{\text{F}}_{\text{2}}}$, ${\text{Xe}}{{\text{O}}_{\text{2}}}{{\text{F}}_{\text{2}}}$ etc.
Complete step by step answer:
The given compound is a compound of xenon, that is, ${\text{XeO}}{{\text{F}}_{\text{2}}}$. Here, the xenon is bonded to one oxygen atom and two fluorine atoms.
We know that xenon is a noble gas that belongs to the group 18 of the periodic table. Its atomic number is 54. The electronic configuration of xenon is $\left[ {{\text{Kr}}} \right]4{d^{10}}5{s^2}5{p^6}$. So, the number of valence electrons of xenon is 8.
Now, we discuss the covalent bonding in ${\text{XeO}}{{\text{F}}_{\text{2}}}$. Out of 8 valence electrons, two are shared with the oxygen atom to form double covalent bond and one electron is shared with two fluorine atoms each to form two Xe-F single covalent bonds. So, four electrons remain unshared on the xenon atom.
Let’s understand with the help of the diagram.

So, the number of lone pairs in ${\text{XeO}}{{\text{F}}_{\text{2}}}$ is 2.
Note: It is to be remembered that all elements belong to group 18 are termed as noble gas. These elements have a complete octet of valence shell, so they are inert in nature. The best characterized compounds are formed due to the reaction of xenon with oxygen and fluorine under certain conditions. Some example of xenon compounds are ${\text{XeO}}{{\text{F}}_{\text{2}}}$, ${\text{Xe}}{{\text{O}}_{\text{2}}}{{\text{F}}_{\text{2}}}$ etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




