
List a few properties of benzene diazonium chloride.
Answer
514.2k+ views
Hint: Diazonium salts are those compounds possessing Azo group $ - N = N - $ (di means two and the word Azo is derived from French word azote=Nitrogen).They have a general formula $Ar - {N_2}X$ where $X$ is $Cl,Br,I,N{O_3}$. Peter Griess was the first scientist to who discovered these salts in 1858 in Hofmann’s Laboratory.
Complete answer:
Benzene diazonium chloride has the following structure-
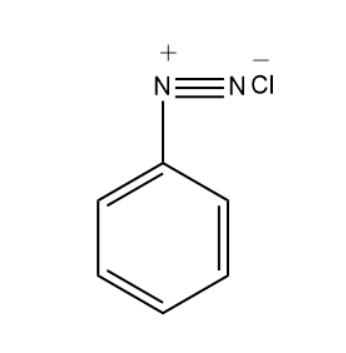
Preparation of Benzene Diazonium Chloride-
Benzene Diazonium chloride is prepared by the reaction of an Aromatic primary amine, Aniline, with Nitric acid solution. The temperature is maintained below 5 °C.This reaction is called diazotization reaction.
$
NaN{O_2} + HCl \to HN{O_2} + NaCl \\
{C_6}{H_5}N{H_2} + HCl \to {C_6}{H_5}N{H_3}Cl \\
{C_6}{H_5}N{H_3}Cl + HN{O_2} \to {C_6}{H_5}{N_2}Cl + 2{H_2}O \\
\\
$
Physical properties of Benzene Diazonium Chloride-
Benzene Diazonium salts are colourless crystalline solids
It is readily soluble in water but sparingly soluble in alcohol and ether.
On Exposure to air they turn brown.
They are extremely unstable compounds as they explode violently when heated or detonated.
It is the parent member of aryl diazonium compound which is widely used in organic chemistry.
Chemical properties of Benzene Diazonium Chloride-
Some important reactions are as under-
1. Replacement by Hydrogen-
${C_6}{H_5}{N_2}Cl + {H_3}P{O_2}\xrightarrow{{C{u^ + }}}{C_6}{H_6} + {H_3}P{O_3} + {N_2} + HCl$
When aqueous solution of diazonium salt is treated with hypophosphorous acid in presence of cuprous chloride it forms benzene.
2. Replacement by Hydroxyl group-
${C_6}{H_5}{N_2}Cl + {H_2}O\xrightarrow{{{H_2}S{O_4}}}{C_6}{H_5}OH + {N_2} + HCl$
Aqueous solution of diazonium salt with a few drops of sulphuric acid on heating produces phenols.
3. Replacement by halogens-
$\begin{gathered}
{C_6}{H_5}{N_2}Cl\xrightarrow{{C{u_2}C{l_2}/HCl}}{C_6}{H_5}Cl + {N_2} \\
2{C_6}{H_5}{N_2}Cl\xrightarrow{{C{u_2}B{r_2}/HBr}}2{C_6}{H_5}Br + 2{N_2} + C{u_2}C{l_2} \\
\end{gathered} $\[\]
When aqueous solution of diazonium chloride is added to the concentrated solution of cuprous halide in corresponding halogen acid, it produces aryl halides.
4. Replacement by cyano group-
${C_6}{H_5}{N_2}Cl\xrightarrow{{CuCN/KCN}}{C_6}{H_5}CN + CuCN + {N_2}$
This reaction occurs on reaction of diazonium salt with cuprous cyanide dissolved in potassium cyanide.
5. Replacement by Nitro group-
${C_6}{H_5}{N_2}Cl + HN{O_2}\xrightarrow{{C{u_2}O}}{C_6}{H_5}N{O_2} + {N_2} + HCl$
This reaction occurs on reaction of diazonium salt with nitrous acid in presence of cuprous oxide.
6. Replacement by amino group-
${C_6}{H_5}{N_2}Cl + {(N{H_4})_2}C{O_3} \to {C_6}{H_5}N{H_2} + N{H_4}Cl + C{O_2} + {N_2} + {H_2}O$
On boiling of diazonium chloride with Ammonium carbonate we obtain Aniline.
7. Reduction-
${C_6}{H_5}{N_2}Cl + 4H\xrightarrow{{SnC{l_2} + HCl}}{C_6}{H_5}NH - N{H_2}HCl$
Diazonium salt on reaction with $SnC{l_2}/HCl$ yields phenyl hydrazine.
8. Coupling Reaction-
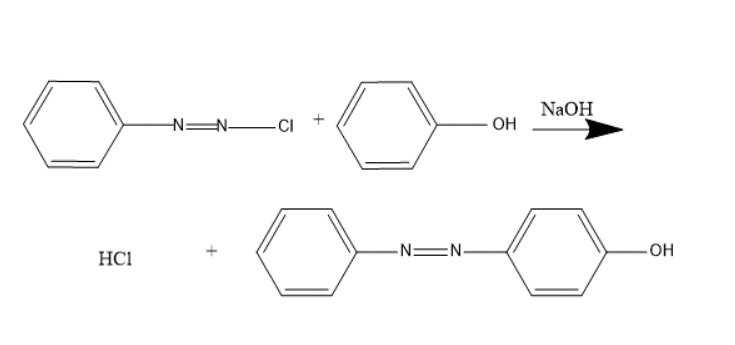
Diazonium salts react with compounds containing labile hydrogen (phenol, aniline and its derivative) to produce azo compounds. It is employed in preparation of a number of dyes. Benzene diazonium chloride reacts with phenol in a slightly alkaline solution to give the product p-Hydroxy Azo benzene which is an orange dye. In coupling, attachment of azo at ortho or para positions takes place.
Note:
These diazonium chlorides find wide applications in the dye and pigment industry. These are useful in synthesis of large numbers of organic compounds. Also they find use in document reproduction due to the property to break down in U.V light.
Complete answer:
Benzene diazonium chloride has the following structure-

Preparation of Benzene Diazonium Chloride-
Benzene Diazonium chloride is prepared by the reaction of an Aromatic primary amine, Aniline, with Nitric acid solution. The temperature is maintained below 5 °C.This reaction is called diazotization reaction.
$
NaN{O_2} + HCl \to HN{O_2} + NaCl \\
{C_6}{H_5}N{H_2} + HCl \to {C_6}{H_5}N{H_3}Cl \\
{C_6}{H_5}N{H_3}Cl + HN{O_2} \to {C_6}{H_5}{N_2}Cl + 2{H_2}O \\
\\
$
Physical properties of Benzene Diazonium Chloride-
Benzene Diazonium salts are colourless crystalline solids
It is readily soluble in water but sparingly soluble in alcohol and ether.
On Exposure to air they turn brown.
They are extremely unstable compounds as they explode violently when heated or detonated.
It is the parent member of aryl diazonium compound which is widely used in organic chemistry.
Chemical properties of Benzene Diazonium Chloride-
Some important reactions are as under-
1. Replacement by Hydrogen-
${C_6}{H_5}{N_2}Cl + {H_3}P{O_2}\xrightarrow{{C{u^ + }}}{C_6}{H_6} + {H_3}P{O_3} + {N_2} + HCl$
When aqueous solution of diazonium salt is treated with hypophosphorous acid in presence of cuprous chloride it forms benzene.
2. Replacement by Hydroxyl group-
${C_6}{H_5}{N_2}Cl + {H_2}O\xrightarrow{{{H_2}S{O_4}}}{C_6}{H_5}OH + {N_2} + HCl$
Aqueous solution of diazonium salt with a few drops of sulphuric acid on heating produces phenols.
3. Replacement by halogens-
$\begin{gathered}
{C_6}{H_5}{N_2}Cl\xrightarrow{{C{u_2}C{l_2}/HCl}}{C_6}{H_5}Cl + {N_2} \\
2{C_6}{H_5}{N_2}Cl\xrightarrow{{C{u_2}B{r_2}/HBr}}2{C_6}{H_5}Br + 2{N_2} + C{u_2}C{l_2} \\
\end{gathered} $\[\]
When aqueous solution of diazonium chloride is added to the concentrated solution of cuprous halide in corresponding halogen acid, it produces aryl halides.
4. Replacement by cyano group-
${C_6}{H_5}{N_2}Cl\xrightarrow{{CuCN/KCN}}{C_6}{H_5}CN + CuCN + {N_2}$
This reaction occurs on reaction of diazonium salt with cuprous cyanide dissolved in potassium cyanide.
5. Replacement by Nitro group-
${C_6}{H_5}{N_2}Cl + HN{O_2}\xrightarrow{{C{u_2}O}}{C_6}{H_5}N{O_2} + {N_2} + HCl$
This reaction occurs on reaction of diazonium salt with nitrous acid in presence of cuprous oxide.
6. Replacement by amino group-
${C_6}{H_5}{N_2}Cl + {(N{H_4})_2}C{O_3} \to {C_6}{H_5}N{H_2} + N{H_4}Cl + C{O_2} + {N_2} + {H_2}O$
On boiling of diazonium chloride with Ammonium carbonate we obtain Aniline.
7. Reduction-
${C_6}{H_5}{N_2}Cl + 4H\xrightarrow{{SnC{l_2} + HCl}}{C_6}{H_5}NH - N{H_2}HCl$
Diazonium salt on reaction with $SnC{l_2}/HCl$ yields phenyl hydrazine.
8. Coupling Reaction-
Diazonium salts react with compounds containing labile hydrogen (phenol, aniline and its derivative) to produce azo compounds. It is employed in preparation of a number of dyes. Benzene diazonium chloride reacts with phenol in a slightly alkaline solution to give the product p-Hydroxy Azo benzene which is an orange dye. In coupling, attachment of azo at ortho or para positions takes place.

Note:
These diazonium chlorides find wide applications in the dye and pigment industry. These are useful in synthesis of large numbers of organic compounds. Also they find use in document reproduction due to the property to break down in U.V light.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

Which out of the following hydrocarbons undergo addition class 11 chemistry CBSE




