
What is latent heat and triple point?
Answer
522.9k+ views
Hint: The amount of heat radiated or absorbed during the transformation phase of a substance at constant temperature and pressure is known as latent heat. The temperature and pressure at which all three phases coexist is known as triple point. A triple point is unique for every substance.
Complete step-by-step solution:
Latent heat
Example - The heat of freezing and the heat of vaporization.
The heat of freezing is the amount of thermal energy discharged when a liquid freezes and the heat of vaporization is the amount of thermal energy that must be added up to transform the liquid into gas.
The latent heat formula is given as,
\[Q = M \times L\]
Where, \[Q\] is the amount of heat? \[M\] Is the mass of the substance, The Latent Heat is represented by \[L\]. The unit of latent heat is \[J/kg\].
The above equation states that the heat ‘Q’ must either be subtracted or added up in the object of mass ‘M’ to transform the phases.
The values of latent heat are variable and rely on the occurrence of the transformation phase- The latent heat of fusion implies the transformation of a liquid to a solid, The latent heat of vaporization implies the change from a liquid to a gas, The latent heat of sublimation implies the change from a solid to a gas.
Triple point of water
Based on the definition of the triple point of a substance determine the conditions to be satisfied for the triple point of water.
We know the three phases of water are water, steam and ice.
Lowering the temperature causes steam to condense and become water and if the temperature is again lowered it solidifies to form ice.
The temperature at which water boils is \[{100^ \circ }C\] and the temperature at which it freezes is \[{0^ \circ }C\].
At the triple point, all three phases of water must coexist i.e., ice, water and steam have to coexist. For this, the boiling point and freezing point of water has to be the same.
The graph given below depicts the triple point of water.
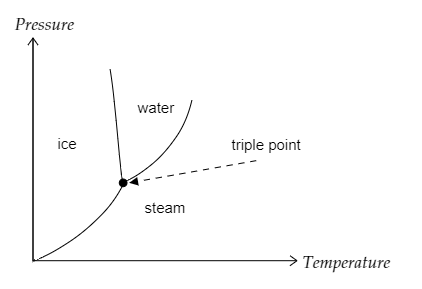
Note: It is a very important point to remember that the latent heat of fusion is defined for a constant pressure and this pressure is \[1atm = 1.01325 \times {10^5}Pa\], which the normal atmospheric pressure is at sea level. This is because melting points and the heat required changes upon the pressure of the surroundings and since the latent heat of fusion is a characteristic property of a substance, it must be defined as a constant and therefore, be defined at a specific pressure.
Complete step-by-step solution:
Latent heat
Example - The heat of freezing and the heat of vaporization.
The heat of freezing is the amount of thermal energy discharged when a liquid freezes and the heat of vaporization is the amount of thermal energy that must be added up to transform the liquid into gas.
The latent heat formula is given as,
\[Q = M \times L\]
Where, \[Q\] is the amount of heat? \[M\] Is the mass of the substance, The Latent Heat is represented by \[L\]. The unit of latent heat is \[J/kg\].
The above equation states that the heat ‘Q’ must either be subtracted or added up in the object of mass ‘M’ to transform the phases.
The values of latent heat are variable and rely on the occurrence of the transformation phase- The latent heat of fusion implies the transformation of a liquid to a solid, The latent heat of vaporization implies the change from a liquid to a gas, The latent heat of sublimation implies the change from a solid to a gas.
Triple point of water
Based on the definition of the triple point of a substance determine the conditions to be satisfied for the triple point of water.
We know the three phases of water are water, steam and ice.
Lowering the temperature causes steam to condense and become water and if the temperature is again lowered it solidifies to form ice.
The temperature at which water boils is \[{100^ \circ }C\] and the temperature at which it freezes is \[{0^ \circ }C\].
At the triple point, all three phases of water must coexist i.e., ice, water and steam have to coexist. For this, the boiling point and freezing point of water has to be the same.
The graph given below depicts the triple point of water.

Note: It is a very important point to remember that the latent heat of fusion is defined for a constant pressure and this pressure is \[1atm = 1.01325 \times {10^5}Pa\], which the normal atmospheric pressure is at sea level. This is because melting points and the heat required changes upon the pressure of the surroundings and since the latent heat of fusion is a characteristic property of a substance, it must be defined as a constant and therefore, be defined at a specific pressure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




