
How can I know how many hydrogens are there for each carbon in bond line notations?
Answer
558.9k+ views
Hint: Carbon has four valence electrons in its outer shell and carbon is going to form four bonds with other atoms. Hydrogen has one valence electron and hydrogen is going to form a single bond with other atoms.
Complete step by step answer:
- In the question it is asked how we can know how many hydrogens are there on each carbon in a bond line notation.
- Bond line notation is a simple way to represent the simple organic compounds.
- In bond line notation there is involvement of carbon and hydrogen representation in the organic molecule and it can be represented by taking an example of propane and it is as follows.
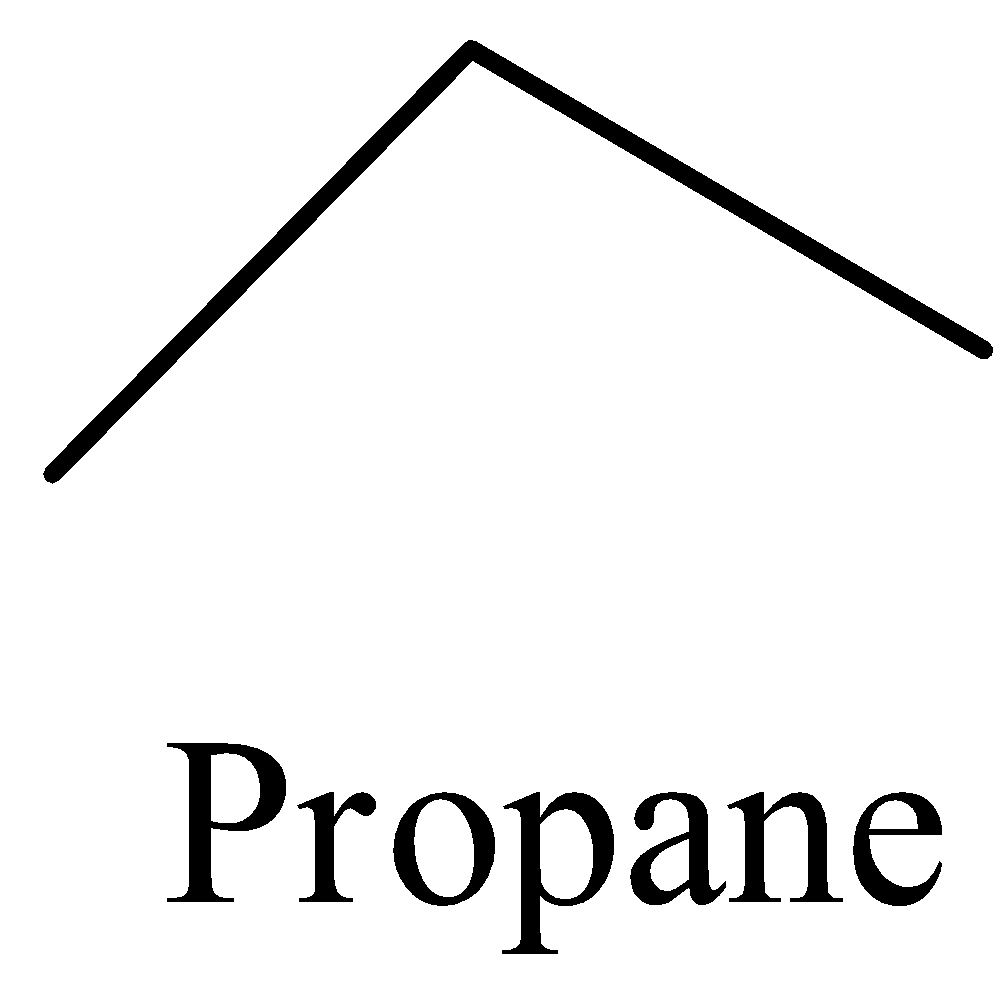
- Now coming to the main concept we can find how many hydrogens are there on each carbon atom in bond line notation.
- We know that carbon is going to form four bonds with other atoms.
- In bond line notation first we have to calculate how bonds are there with one carbon.
- For example take propane :
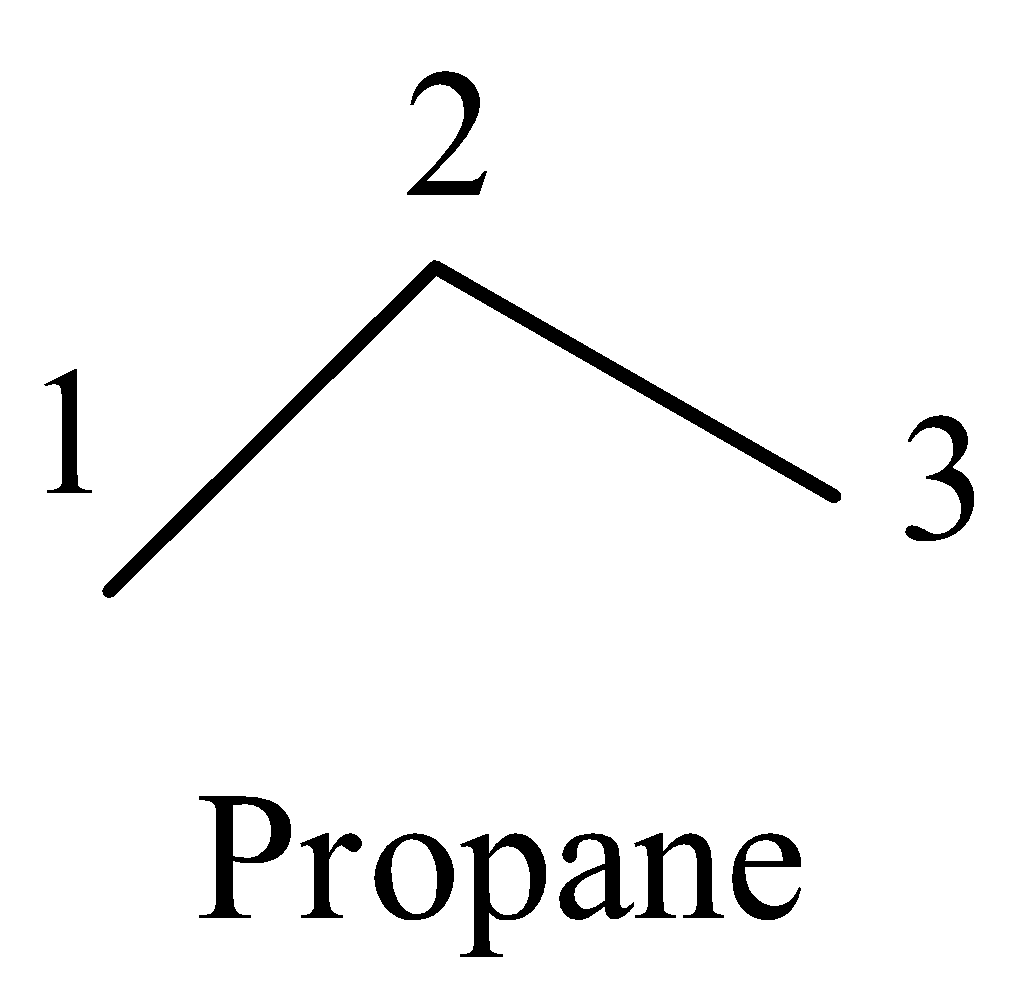
- The carbon one has one bond with carbon two means the remaining three valence electrons of carbon are going to form three bonds with three hydrogen atoms. Means on carbon one there are three hydrogens.
- Coming to carbon-2, it is bonded to carbon-1 and carbon-3, then the remaining two valence electrons are going to form bonds with two more hydrogens than the carbon-2 has two hydrogens on it.
- This is how we can calculate the number of hydrogens on carbon in bond line notation.
Note: It is very easy to find the number of hydrogens on the carbon in bond line notation, just we are supposed to do it if we have to find that the carbon atom has how many bonds with the other carbon atoms in the given molecule.
Complete step by step answer:
- In the question it is asked how we can know how many hydrogens are there on each carbon in a bond line notation.
- Bond line notation is a simple way to represent the simple organic compounds.
- In bond line notation there is involvement of carbon and hydrogen representation in the organic molecule and it can be represented by taking an example of propane and it is as follows.

- Now coming to the main concept we can find how many hydrogens are there on each carbon atom in bond line notation.
- We know that carbon is going to form four bonds with other atoms.
- In bond line notation first we have to calculate how bonds are there with one carbon.
- For example take propane :

- The carbon one has one bond with carbon two means the remaining three valence electrons of carbon are going to form three bonds with three hydrogen atoms. Means on carbon one there are three hydrogens.
- Coming to carbon-2, it is bonded to carbon-1 and carbon-3, then the remaining two valence electrons are going to form bonds with two more hydrogens than the carbon-2 has two hydrogens on it.
- This is how we can calculate the number of hydrogens on carbon in bond line notation.
Note: It is very easy to find the number of hydrogens on the carbon in bond line notation, just we are supposed to do it if we have to find that the carbon atom has how many bonds with the other carbon atoms in the given molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




