
How is the kinetic energy versus temperature graph for a given substance?

Answer
579k+ views
Hint: Write the relationship between the average kinetic energy of the molecules and absolute temperature. From this relationship, decide if the plot is a straight line or a curve. For a curve, decide the shape of the curve from the relationship. If a straight line is obtained, then decide if the slope is positive or negative.
Complete Step by step answer: The average kinetic energy of the molecules is \[{\text{K}}{\text{.E = }}\dfrac{3}{2}{\text{kT }}\]
Here, \[{\text{K}}{\text{.E}}\] represents the average kinetic energy of the molecules, \[{\text{k}}\] represents the Boltzmann constant whereas \[{\text{T}}\] represents the absolute temperature.
Thus, the average kinetic energy of the molecules is directly proportional to the absolute temperature.
\[{\text{K}}{\text{.E }} \propto {\text{T}}\]
At absolute zero, the average kinetic energy of the molecules has a value of zero. With increase in the absolute temperature, the average kinetic energy of the molecules also increases.
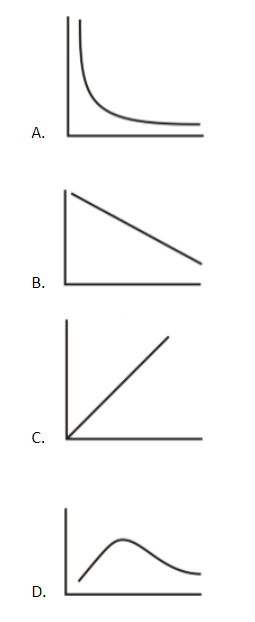
For one mole of the ideal gas, the average molecular kinetic energy of molecules is plotted on the Y axis whereas the absolute temperature is plotted on the X axis. The plot is a straight line passing through the origin and having a positive slope. Such a plot is obtained in the plot of the option (C).
Hence, the correct option is the option (C).
Note: We should note that the kinetic energy is expressed in terms of temperature and not the velocity. So we can also say that there would be relation in between the temperature and the velocity of molecules.The Boltzmann constant \[{\text{k = }}\dfrac{{\text{R}}}{{{{\text{N}}_{\text{A}}}}}\] .Here, \[{\text{R}}\] is the ideal gas constant and \[{{\text{N}}_{\text{A}}}\] is the Avogadro’s number. The value of the Boltzmann constant is \[{\text{1}}{\text{.38}} \times {\text{1}}{{\text{0}}^{ - {\text{23}}}}{\text{ Joule molecul}}{{\text{e}}^{ - 1}}{\text{ }}{{\text{K}}^{ - 1}}\] .
Complete Step by step answer: The average kinetic energy of the molecules is \[{\text{K}}{\text{.E = }}\dfrac{3}{2}{\text{kT }}\]
Here, \[{\text{K}}{\text{.E}}\] represents the average kinetic energy of the molecules, \[{\text{k}}\] represents the Boltzmann constant whereas \[{\text{T}}\] represents the absolute temperature.
Thus, the average kinetic energy of the molecules is directly proportional to the absolute temperature.
\[{\text{K}}{\text{.E }} \propto {\text{T}}\]
At absolute zero, the average kinetic energy of the molecules has a value of zero. With increase in the absolute temperature, the average kinetic energy of the molecules also increases.
For one mole of the ideal gas, the average molecular kinetic energy of molecules is plotted on the Y axis whereas the absolute temperature is plotted on the X axis. The plot is a straight line passing through the origin and having a positive slope. Such a plot is obtained in the plot of the option (C).
Hence, the correct option is the option (C).
Note: We should note that the kinetic energy is expressed in terms of temperature and not the velocity. So we can also say that there would be relation in between the temperature and the velocity of molecules.The Boltzmann constant \[{\text{k = }}\dfrac{{\text{R}}}{{{{\text{N}}_{\text{A}}}}}\] .Here, \[{\text{R}}\] is the ideal gas constant and \[{{\text{N}}_{\text{A}}}\] is the Avogadro’s number. The value of the Boltzmann constant is \[{\text{1}}{\text{.38}} \times {\text{1}}{{\text{0}}^{ - {\text{23}}}}{\text{ Joule molecul}}{{\text{e}}^{ - 1}}{\text{ }}{{\text{K}}^{ - 1}}\] .
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE




