
What kind of reaction breaks down polymers into monomers?
Answer
526.8k+ views
Hint: The monomers like amino acids combined through a peptide bond to form a polypeptide chain and later the polypeptide chains combined and forms a protein structure. At the same time the polypeptide chain undergoes breakdown and forms monomers amino acids as the products.
Complete answer:
- In the question it is asked what type of reaction breaks down the polymers into monomers.
- Almost the formation of all the polymers occurs through the release of the water molecules as the byproduct.
- By using the same byproduct we can break the formed bond and the chemical reaction is called hydrolysis.
- For example take a dimer of carbohydrate and there is a glycosidic bond in between the glucose and galactose.
- The cleavage or the hydrolysis of lactose is going to give two monomers called glucose and galactose.
- We can see the hydrolysis of the lactose means conversion of a dimer into two monomers as shown in below chemical reaction hydrolysis of lactose.
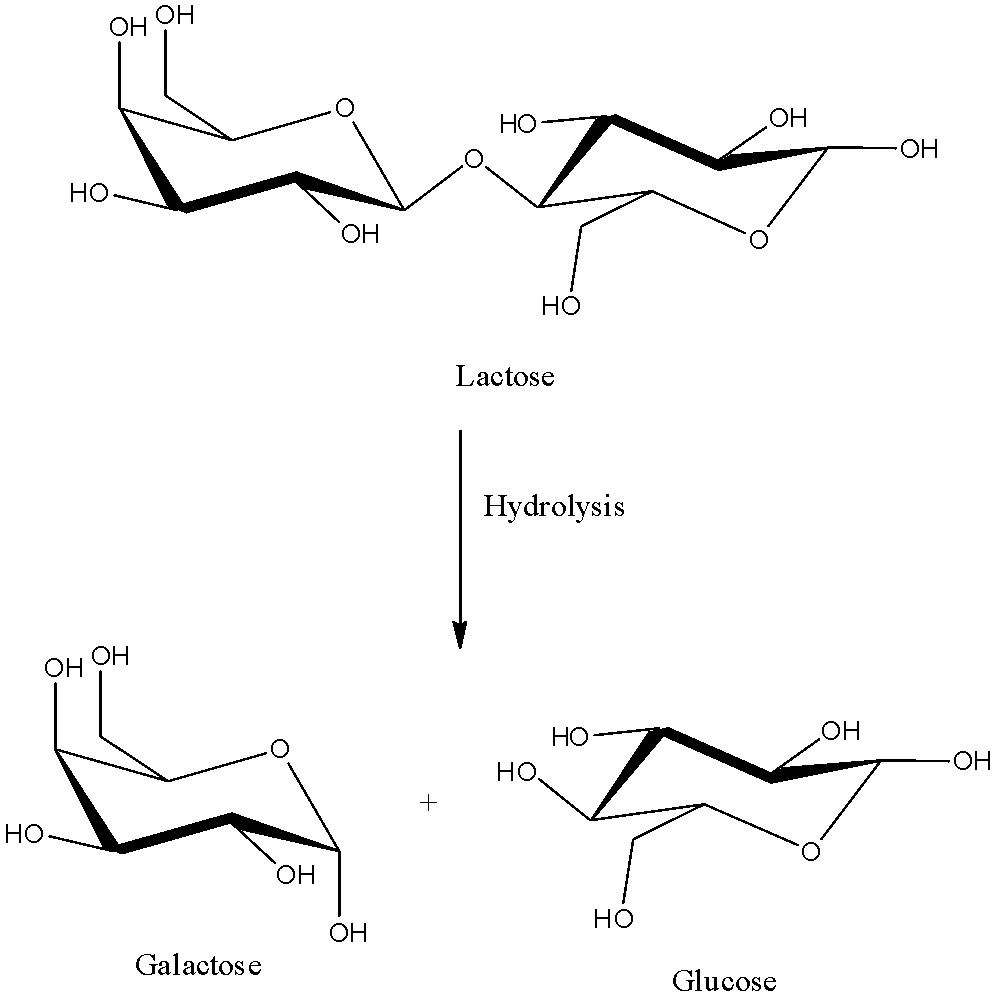
- Hydrolysis is going to break the bond between the monomers in the polymeric chain.
- The process of breakdown of the polymers into monomers is called hydrolysis or the cleavage of the bond between the monomers in a polymeric chain.
Note:
The opposite to hydrolysis is dehydration which is going to remove the water and forms a bond between the monomers to form the process of the preparation of the polymer and the process is called polymerization.
Complete answer:
- In the question it is asked what type of reaction breaks down the polymers into monomers.
- Almost the formation of all the polymers occurs through the release of the water molecules as the byproduct.
- By using the same byproduct we can break the formed bond and the chemical reaction is called hydrolysis.
- For example take a dimer of carbohydrate and there is a glycosidic bond in between the glucose and galactose.
- The cleavage or the hydrolysis of lactose is going to give two monomers called glucose and galactose.
- We can see the hydrolysis of the lactose means conversion of a dimer into two monomers as shown in below chemical reaction hydrolysis of lactose.

- Hydrolysis is going to break the bond between the monomers in the polymeric chain.
- The process of breakdown of the polymers into monomers is called hydrolysis or the cleavage of the bond between the monomers in a polymeric chain.
Note:
The opposite to hydrolysis is dehydration which is going to remove the water and forms a bond between the monomers to form the process of the preparation of the polymer and the process is called polymerization.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




