
What is the IUPAC name for isophthalic acid?
(a) Benzene-1, 3-dicarboxylic acid
(b) Benzene-1, 2-dicarboxylic acid
(c) Benzene-1, 4-dicarboxylic acid
(d) Benzene-1, 5-dicarboxylic acid
Answer
586.2k+ views
Hint: The chemical formula of isophthalic acid is ${{C}_{8}}{{H}_{6}}{{O}_{4}}$ and there are two carboxylic acid groups attached to the carbon atom in the benzene ring at 1st and 3rd position.
Complete step by step answer:
-Isophthalic acid is an organic compound in which two carboxylic acid groups are attached to the benzene ring. The chemical formula of isophthalic acid is ${{C}_{8}}{{H}_{6}}{{O}_{4}}$. The two carboxylic acid groups attached to the carbon atom in the benzene ring at 1st and 3rd position.
-So the structure of the isophthalic acid is given below:
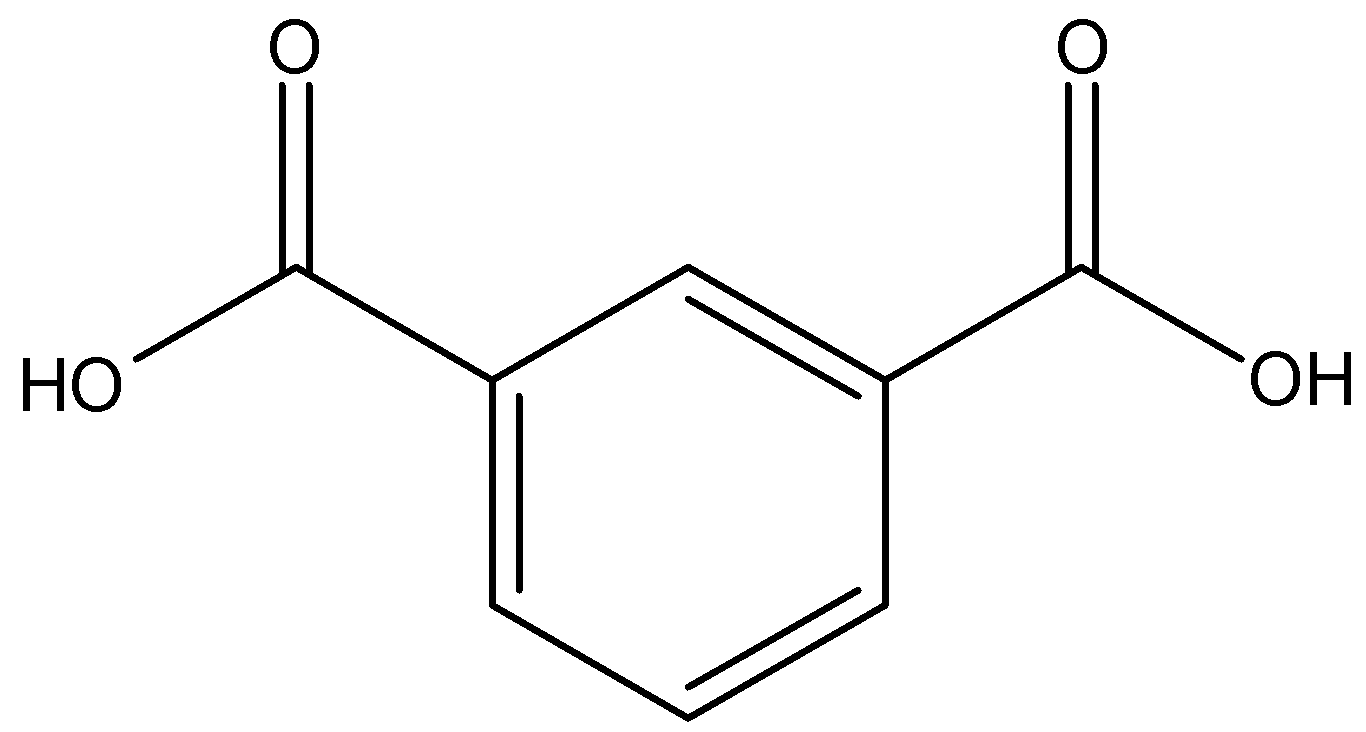
-So the IUPAC name of isophthalic acid is Benzene-1, 3-dicarboxylic acid.
-The molecular mass of Benzene-1, 3-dicarboxylic acid is 166.132 g / mol.
-The appearance of Benzene-1, 3-dicarboxylic acid is white crystalline solid.
-The density of Benzene-1, 3-dicarboxylic acid is $1.526\text{ g/c}{{\text{m}}^{3}}$.
-Benzene-1, 3-dicarboxylic acid is not soluble in water.
-It is one of the three isomers of benzene dicarboxylic acid, the other two are phthalic acid and terephthalic acid. Phthalic acid is the ortho-isomer and Terephthalic acid is the para-isomer.
-Benzene-1, 3-dicarboxylic acid is the meta-isomer.
-Benzene-1, 3-dicarboxylic acid is easily and produced in a huge amount by oxidizing meta-xylene using oxygen.
-Lotte Chemical Corporation is the company that produced the largest amount of isophthalic acid. It is mainly used for making polyethylene terephthalate (PET).
So the correct answer is an option (a) Benzene-1, 3-dicarboxylic acid.
Note: Since there are three isomers of Benzene dicarboxylic acid, isophthalic is Benzene-1, 3-dicarboxylic acid, phthalic acid is Benzene-1, 2-dicarboxylic acid, and terephthalic acid is Benzene-1, 4-dicarboxylic acid, so don’t get confused between them.
Complete step by step answer:
-Isophthalic acid is an organic compound in which two carboxylic acid groups are attached to the benzene ring. The chemical formula of isophthalic acid is ${{C}_{8}}{{H}_{6}}{{O}_{4}}$. The two carboxylic acid groups attached to the carbon atom in the benzene ring at 1st and 3rd position.
-So the structure of the isophthalic acid is given below:

-So the IUPAC name of isophthalic acid is Benzene-1, 3-dicarboxylic acid.
-The molecular mass of Benzene-1, 3-dicarboxylic acid is 166.132 g / mol.
-The appearance of Benzene-1, 3-dicarboxylic acid is white crystalline solid.
-The density of Benzene-1, 3-dicarboxylic acid is $1.526\text{ g/c}{{\text{m}}^{3}}$.
-Benzene-1, 3-dicarboxylic acid is not soluble in water.
-It is one of the three isomers of benzene dicarboxylic acid, the other two are phthalic acid and terephthalic acid. Phthalic acid is the ortho-isomer and Terephthalic acid is the para-isomer.
-Benzene-1, 3-dicarboxylic acid is the meta-isomer.
-Benzene-1, 3-dicarboxylic acid is easily and produced in a huge amount by oxidizing meta-xylene using oxygen.
-Lotte Chemical Corporation is the company that produced the largest amount of isophthalic acid. It is mainly used for making polyethylene terephthalate (PET).
So the correct answer is an option (a) Benzene-1, 3-dicarboxylic acid.
Note: Since there are three isomers of Benzene dicarboxylic acid, isophthalic is Benzene-1, 3-dicarboxylic acid, phthalic acid is Benzene-1, 2-dicarboxylic acid, and terephthalic acid is Benzene-1, 4-dicarboxylic acid, so don’t get confused between them.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




