
How many isomers of ${{C}_{4}}{{H}_{10}}O$ react with $C{{H}_{3}}MgBr$ to evolve $C{{H}_{4}}$ gas? (Excluding stereoisomer)
Answer
535.6k+ views
Hint: Grignard’s reagents $RMgX$ are very reactive organometallic compounds with carbon-metal bonds. They can react with acidic hydrogen of alcohols to give corresponding hydrocarbons. A general chemical equation for reaction between alcohol and Grignard reagent is
\[{{R}^{\delta -}}-M{{g}^{\delta +}}{{X}^{\delta -}}+R{{O}^{\delta -}}-{{H}^{\delta +}}\to R-H+Mg(OR)X\]
Complete answer:
Let us first find the total number of isomers possible with the chemical formula given, i.e. ${{C}_{4}}{{H}_{10}}O$
General formulas for alcohols and ethers can be given as ${{C}_{n}}{{H}_{n+2}}O$. The chemical formula given to us ${{C}_{4}}{{H}_{10}}O$ fits the general chemical formula for both alcohols and ethers as ${{C}_{4}}{{H}_{2\times 4+2}}O$, therefore, we can say the ${{C}_{4}}{{H}_{10}}O$ is an alcohol or ether.
The possible structures of alcohols with ${{C}_{4}}{{H}_{10}}O$ are shown below:
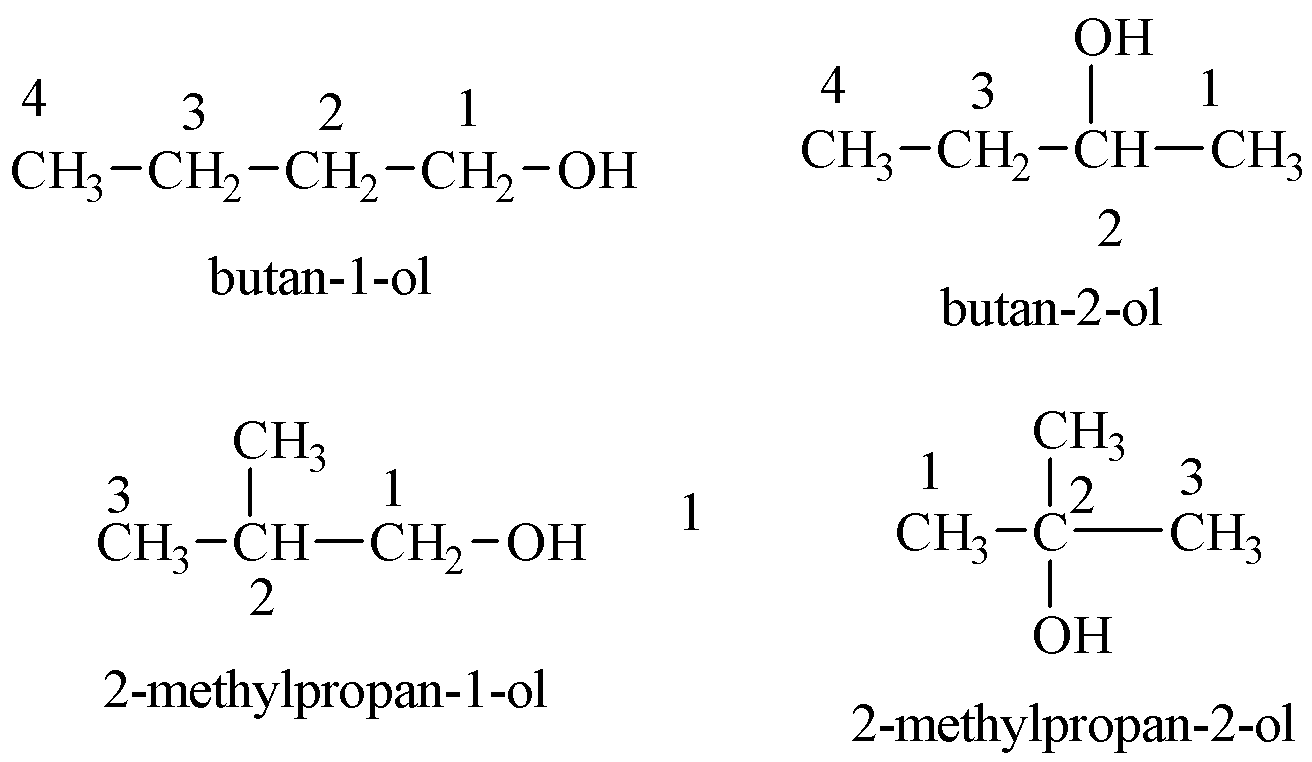
Structures of ethers possible with the chemical formula are given below:
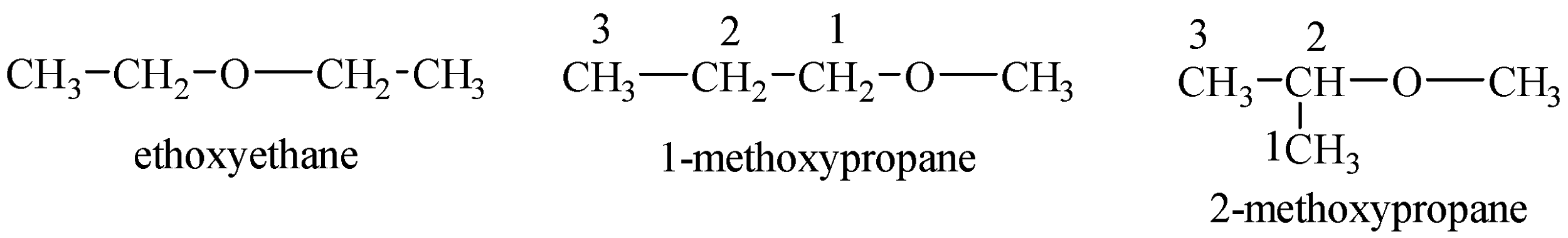
Now, we can see that ethers do not contain any acidic hydrogen and cannot give protons to Grignard’s reagent which is $C{{H}_{3}}MgBr$ in the given question.
Alcohols are acidic in nature due to the polar $O-H$ bond, therefore, can react with Grignard’s reagent $C{{H}_{3}}MgBr$ to give release ($C{{H}_{4}}$) gas. Reactions of all the four alcohols with $C{{H}_{3}}MgBr$ are given below:
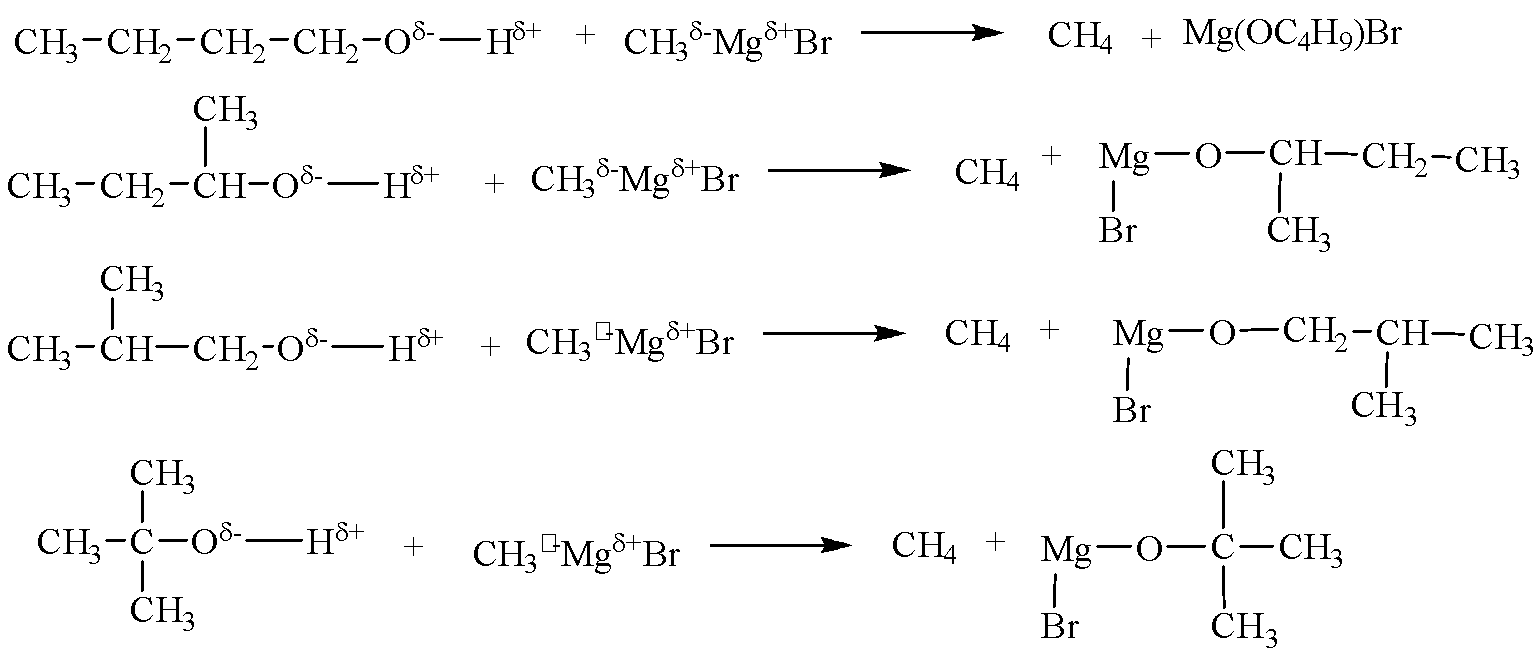
Therefore, four out of the seven isomers of ${{C}_{4}}{{H}_{10}}O$ react with $C{{H}_{3}}MgBr$ to evolve $C{{H}_{4}}$ gas.
So, the correct answer is “Option B”.
Additional Information: One stereoisomer of butan-1-ol is possible as it has a chiral carbon. Stereoisomers have the same molecular and structural formula, i.e. have connectivity of atoms but different spatial arrangement of atoms.
Note: In order to react with $C{{H}_{3}}MgBr$, isomers of ${{C}_{4}}{{H}_{10}}O$ must have an acidic hydrogen. Ethers do not contain any acidic hydrogen as the electronegative O is connected to an alkyl chain, hence, they do not react with Grignard’s reagent.
\[{{R}^{\delta -}}-M{{g}^{\delta +}}{{X}^{\delta -}}+R{{O}^{\delta -}}-{{H}^{\delta +}}\to R-H+Mg(OR)X\]
Complete answer:
Let us first find the total number of isomers possible with the chemical formula given, i.e. ${{C}_{4}}{{H}_{10}}O$
General formulas for alcohols and ethers can be given as ${{C}_{n}}{{H}_{n+2}}O$. The chemical formula given to us ${{C}_{4}}{{H}_{10}}O$ fits the general chemical formula for both alcohols and ethers as ${{C}_{4}}{{H}_{2\times 4+2}}O$, therefore, we can say the ${{C}_{4}}{{H}_{10}}O$ is an alcohol or ether.
The possible structures of alcohols with ${{C}_{4}}{{H}_{10}}O$ are shown below:

Structures of ethers possible with the chemical formula are given below:
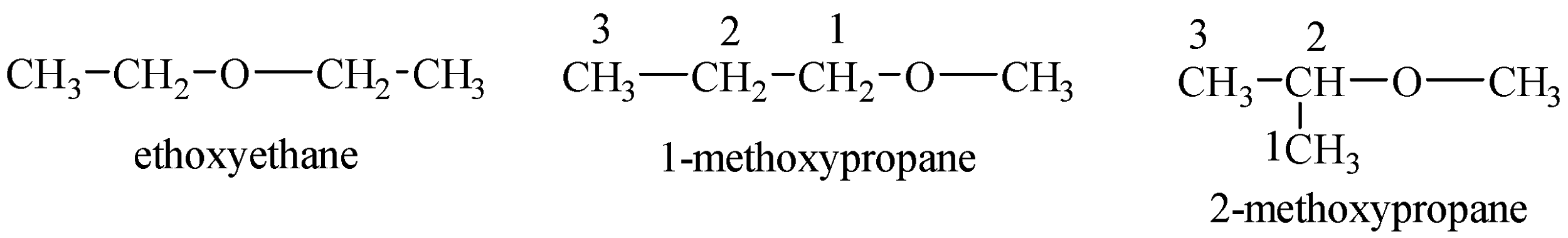
Now, we can see that ethers do not contain any acidic hydrogen and cannot give protons to Grignard’s reagent which is $C{{H}_{3}}MgBr$ in the given question.
Alcohols are acidic in nature due to the polar $O-H$ bond, therefore, can react with Grignard’s reagent $C{{H}_{3}}MgBr$ to give release ($C{{H}_{4}}$) gas. Reactions of all the four alcohols with $C{{H}_{3}}MgBr$ are given below:

Therefore, four out of the seven isomers of ${{C}_{4}}{{H}_{10}}O$ react with $C{{H}_{3}}MgBr$ to evolve $C{{H}_{4}}$ gas.
So, the correct answer is “Option B”.
Additional Information: One stereoisomer of butan-1-ol is possible as it has a chiral carbon. Stereoisomers have the same molecular and structural formula, i.e. have connectivity of atoms but different spatial arrangement of atoms.
Note: In order to react with $C{{H}_{3}}MgBr$, isomers of ${{C}_{4}}{{H}_{10}}O$ must have an acidic hydrogen. Ethers do not contain any acidic hydrogen as the electronegative O is connected to an alkyl chain, hence, they do not react with Grignard’s reagent.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




