
How many isomers are possible for an alkane having molecular formula \[{{C}_{6}}{{H}_{14}}\]?
a.) 3
b.) 4
c.) 5
d.) 6
Answer
600.9k+ views
Hint: Given formula is of alkane. For finding the isomers we draw the different-different structure with the same formula or same number of carbons.
Step by step solution:
We know that each of two or more compounds with the same formula but a different arrangement of atoms in the molecule and different properties are known as isomers. There are three types of structural isomers: chain isomers, functional group isomers and positional isomers.
Functional group isomers have the same molecular formula, but different functional groups on the chain.
Chain isomers are made up of two or more carbon or other compounds with the same molecular formula but different atomic arrangements, or branches.
Positional isomers are constitutional isomers that have the same carbon skeleton and the same functional groups but differ from each other in the location of the functional groups on or in the carbon chain.
Isomers of given formula \[{{C}_{6}}{{H}_{14}}\] are:
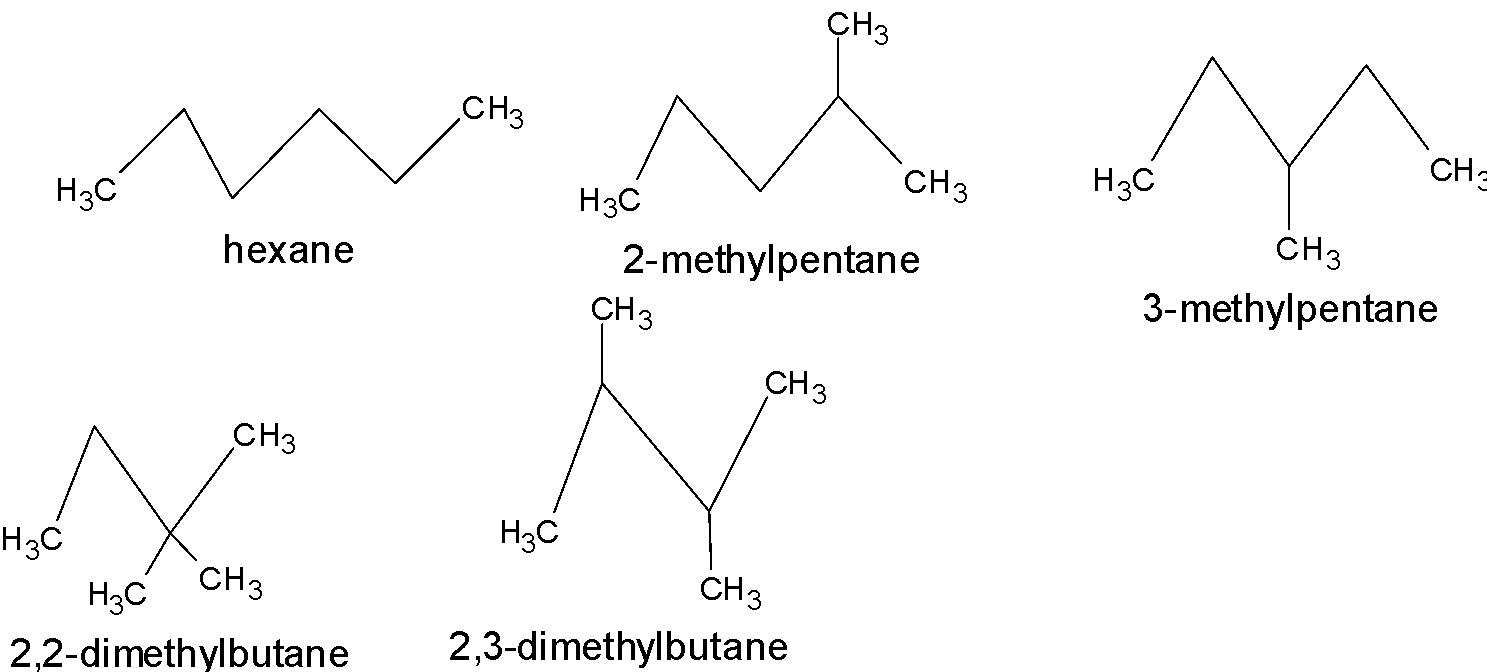 So, there are 5 isomers of \[{{C}_{6}}{{H}_{14}}\].
So, there are 5 isomers of \[{{C}_{6}}{{H}_{14}}\].
The correct answer is option “C”.
Note: In this type of question there will be many confusions when you draw the structures. Those structures look different and can be the same, so you have to write their IUPAC name and then you can easily find that they are similar or different.
Step by step solution:
We know that each of two or more compounds with the same formula but a different arrangement of atoms in the molecule and different properties are known as isomers. There are three types of structural isomers: chain isomers, functional group isomers and positional isomers.
Functional group isomers have the same molecular formula, but different functional groups on the chain.
Chain isomers are made up of two or more carbon or other compounds with the same molecular formula but different atomic arrangements, or branches.
Positional isomers are constitutional isomers that have the same carbon skeleton and the same functional groups but differ from each other in the location of the functional groups on or in the carbon chain.
Isomers of given formula \[{{C}_{6}}{{H}_{14}}\] are:

The correct answer is option “C”.
Note: In this type of question there will be many confusions when you draw the structures. Those structures look different and can be the same, so you have to write their IUPAC name and then you can easily find that they are similar or different.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




