
What is an isomer? Write the isomers of butane with structural formula.
Answer
527.6k+ views
Hint: When there is difference in physical and chemical properties but the number of atoms of each type is the same, it refers to isomerism. The name 'buta’ means 4 i.e. 4 carbon atoms are there in the butane molecule.
Complete step by step answer:
Let us understand the concept of isomerism:
Two or more compounds having the same formula but different chemical and physical properties are called isomers and the phenomenon is called isomerism.
They also have structural differences like different arrangements of the carbon chain, the position of a double bond or functional group, etc.
They also form stereoisomers in which the relative arrangement of atoms or groups in space is different.
Butane is the compound that is a member of the alkane homologous series. Alkanes have only single bonds between the atoms. It is the fourth member of the alkane group. There are 4 carbon atoms in the butane chain. Its molecular formula is \[{{C}_{4}}{{H}_{10}}\]
There are 2 isomers of butane. Let us see both the arrangement:
First is the chain form. There is no branching in this chain form. All the carbons are in a single line. The name of the chain form of an isomer of butane is called n-butane. The structure of n-butane is given below:
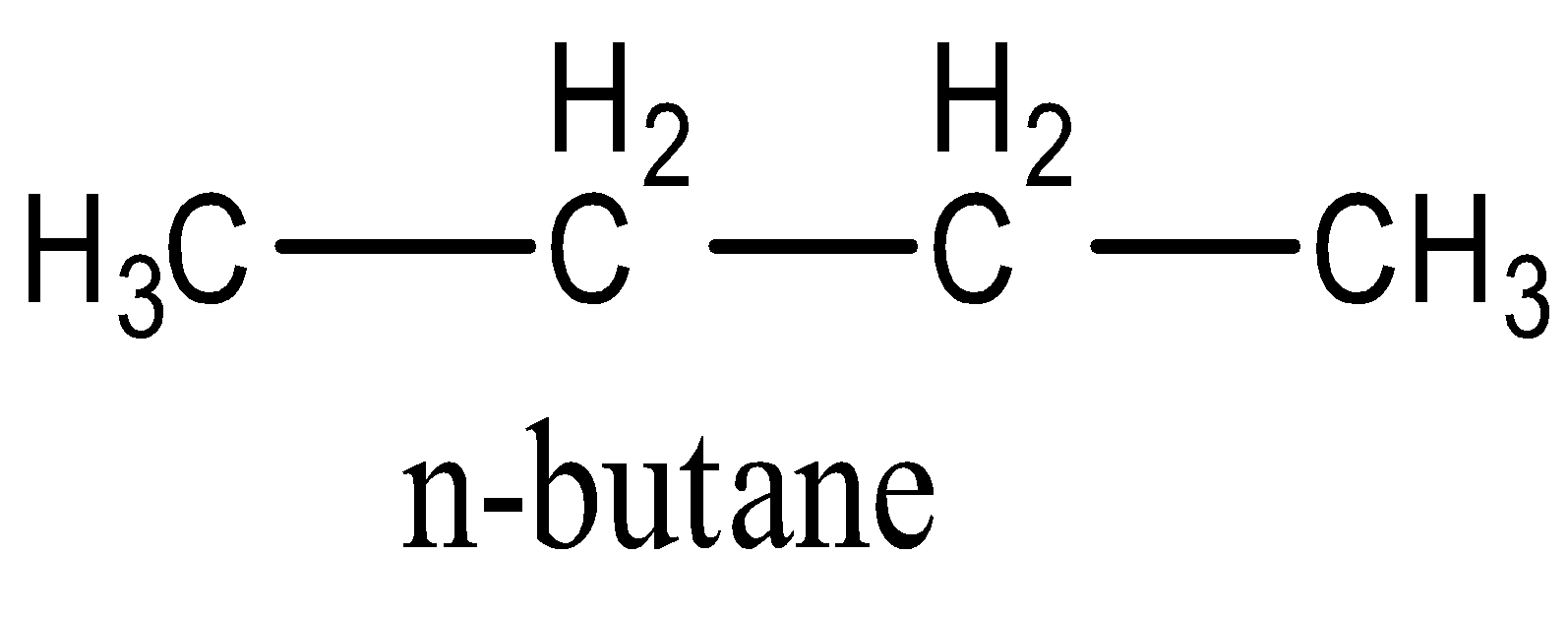
Second is the branched form. There is a branching of one methyl group at the second carbon of the chain. The name of this isomer is 2-methylpropane or its also called isobutene. The structure of isobutene is given below:
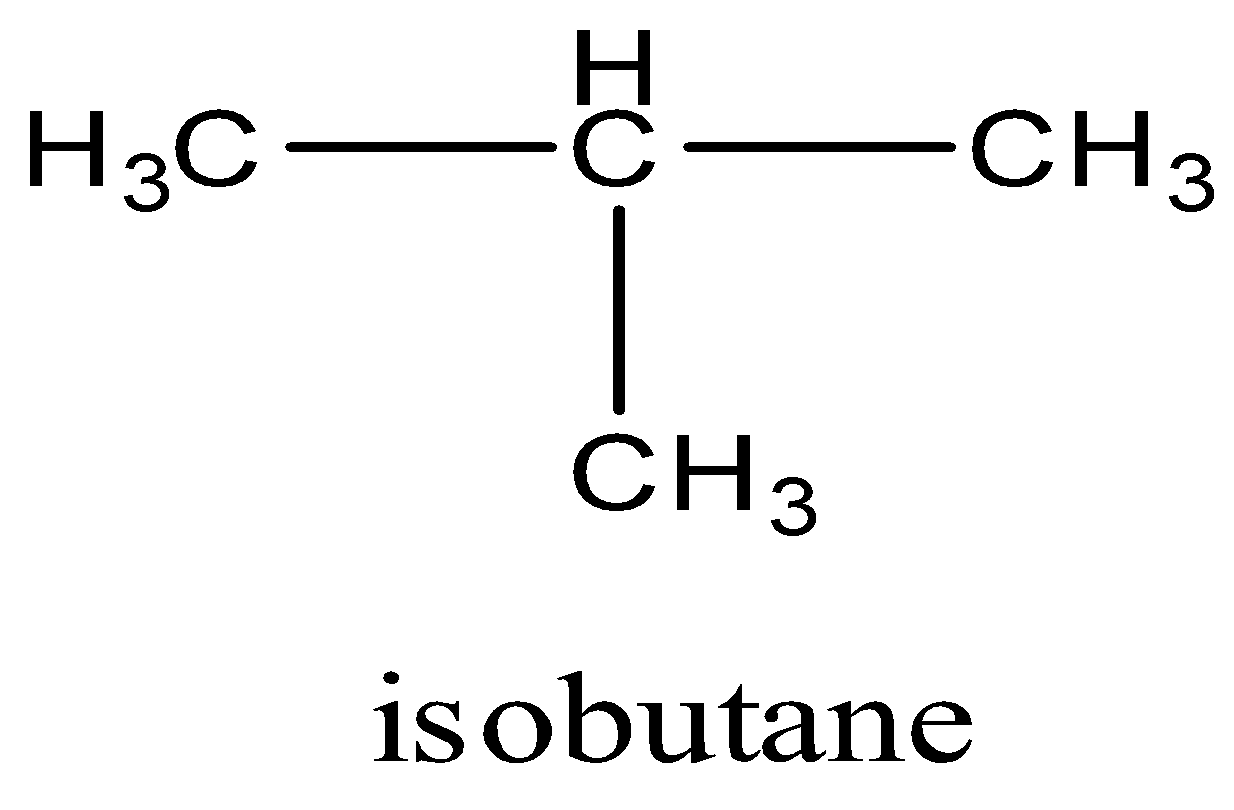
Note:
Whenever the branched-chain molecule is given then the numbering should be given in such a manner that the functional group or the substituent attached to get the least number, otherwise the name of the compound will be wrong. For finding the isomers, every possible structure should be checked.
Complete step by step answer:
Let us understand the concept of isomerism:
Two or more compounds having the same formula but different chemical and physical properties are called isomers and the phenomenon is called isomerism.
They also have structural differences like different arrangements of the carbon chain, the position of a double bond or functional group, etc.
They also form stereoisomers in which the relative arrangement of atoms or groups in space is different.
Butane is the compound that is a member of the alkane homologous series. Alkanes have only single bonds between the atoms. It is the fourth member of the alkane group. There are 4 carbon atoms in the butane chain. Its molecular formula is \[{{C}_{4}}{{H}_{10}}\]
There are 2 isomers of butane. Let us see both the arrangement:
First is the chain form. There is no branching in this chain form. All the carbons are in a single line. The name of the chain form of an isomer of butane is called n-butane. The structure of n-butane is given below:
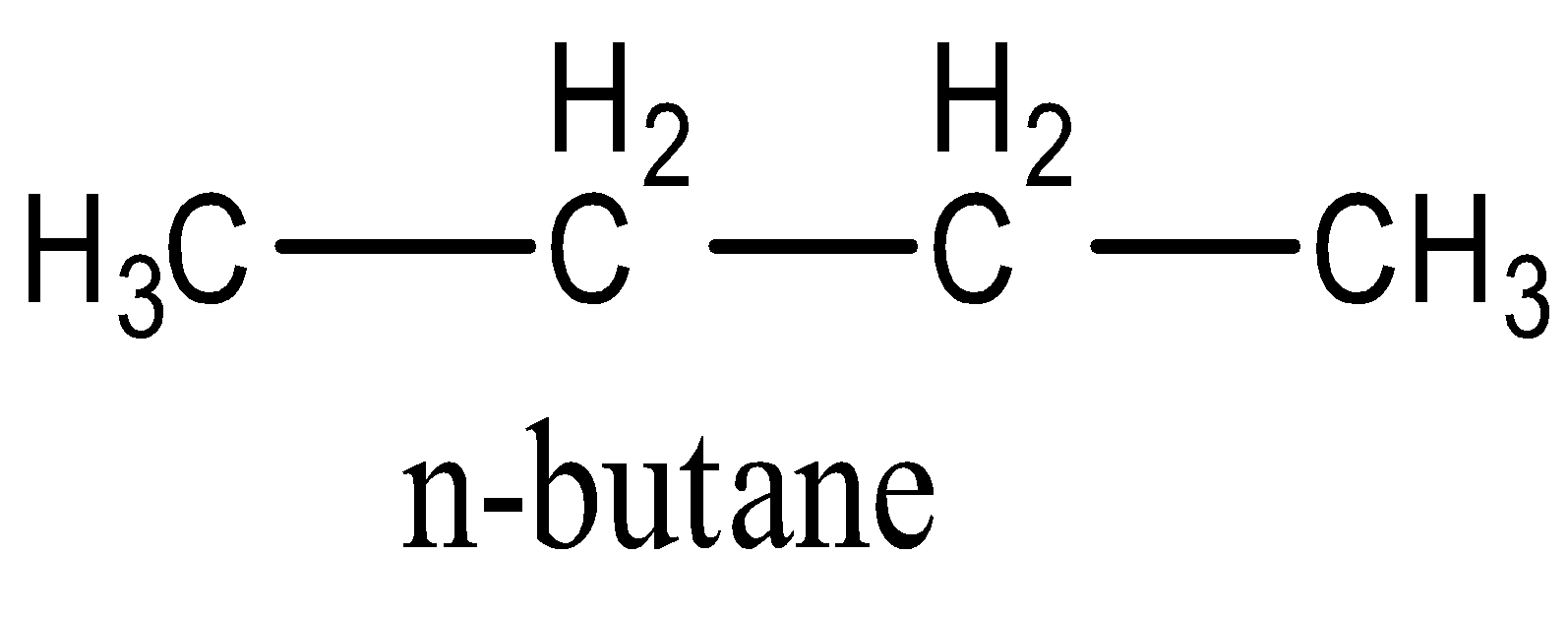
Second is the branched form. There is a branching of one methyl group at the second carbon of the chain. The name of this isomer is 2-methylpropane or its also called isobutene. The structure of isobutene is given below:

Note:
Whenever the branched-chain molecule is given then the numbering should be given in such a manner that the functional group or the substituent attached to get the least number, otherwise the name of the compound will be wrong. For finding the isomers, every possible structure should be checked.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




