
Is water ionic or covalent?
Answer
555.6k+ views
Hint: There are different types of bonds present between the atoms or the molecules which results in formation of different kinds of compounds accordingly.
Some of the bonds which could be differentiated from each other on the basis of the abilities of the atom and the flow of electrons are, ionic bond, covalent bond, hydrogen bond etc.
Complete answer:
An ionic bond is formed when one of the two atoms involved in the bond formation, donates its electrons in order to gain stability, and the other one accepts it for the same reason. The atom which donates electrons are called cations and the atoms which accept them are called anion, after the process of donation. This results in development of positive and negative charge on both the atoms, which leads to formation of attractive forces between them and hence a bond is formed. The atoms which are involved in this kind of bond formation would therefore have different electronegativity from each other. An example of ionic bond is sodium chloride or common salt, where the sodium atom donates the electrons and the chlorine atoms receive it.
On the other hand, a covalent bond is formed as a result of sharing of electrons between two atoms and in a true covalent bond, the electronegativity of both the atoms which are involved in the bond formation, would have equal values and so both the electrons would be in the middle of the bond. In general, there is some electronegativity difference in the atoms involved, and so the electrons would be slightly pulled by that atom having higher electronegativity, which would occasionally result in a polar nature in the bond.
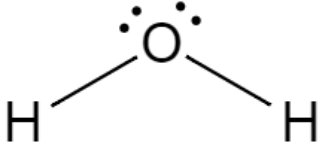
Given above is the diagram of water where we can see one covalent bond is formed between oxygen and each hydrogen.
In the given question, it is asked if water is a covalent or ionic molecule. The correct answer would be, covalent molecule, as it shares electrons between the oxygen and hydrogen atoms.
Note: An ionic bond results from donation of electrons from one atom and acceptance of same electrons from another.
Whereas a covalent bond results from sharing of the electrons among both the atoms.
However, there may be electronegativity difference between the atoms in covalent bonds and so, it could result in a slight polar nature of the bond.
Some of the bonds which could be differentiated from each other on the basis of the abilities of the atom and the flow of electrons are, ionic bond, covalent bond, hydrogen bond etc.
Complete answer:
An ionic bond is formed when one of the two atoms involved in the bond formation, donates its electrons in order to gain stability, and the other one accepts it for the same reason. The atom which donates electrons are called cations and the atoms which accept them are called anion, after the process of donation. This results in development of positive and negative charge on both the atoms, which leads to formation of attractive forces between them and hence a bond is formed. The atoms which are involved in this kind of bond formation would therefore have different electronegativity from each other. An example of ionic bond is sodium chloride or common salt, where the sodium atom donates the electrons and the chlorine atoms receive it.
On the other hand, a covalent bond is formed as a result of sharing of electrons between two atoms and in a true covalent bond, the electronegativity of both the atoms which are involved in the bond formation, would have equal values and so both the electrons would be in the middle of the bond. In general, there is some electronegativity difference in the atoms involved, and so the electrons would be slightly pulled by that atom having higher electronegativity, which would occasionally result in a polar nature in the bond.

Given above is the diagram of water where we can see one covalent bond is formed between oxygen and each hydrogen.
In the given question, it is asked if water is a covalent or ionic molecule. The correct answer would be, covalent molecule, as it shares electrons between the oxygen and hydrogen atoms.
Note: An ionic bond results from donation of electrons from one atom and acceptance of same electrons from another.
Whereas a covalent bond results from sharing of the electrons among both the atoms.
However, there may be electronegativity difference between the atoms in covalent bonds and so, it could result in a slight polar nature of the bond.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




