
Is pyridine an aromatic compound?
Answer
519.3k+ views
Hint :A compound is said to be aromatic if it is flat, cyclic and conjugated and also obeys Huckel’s rule. Huckel's rule states that an organic compound should have pi electrons in the overlapping $ p $ orbitals in order to be aromatic.
Complete Step By Step Answer:
The $ \pi $ orbital system of pyridine has $ p $ electrons which are delocalized throughout the ring. It also has $ 4n + 2 $ delocalized $ p $ electrons, where $ n = 1 $ . Pyridine is planar as all of its $ p $ orbitals are perpendicular to the ring. Also, it is a cyclic compound. It matches all the qualifications of an aromatic compound. So, pyridine is an aromatic compound. The structure of pyridine is given below:
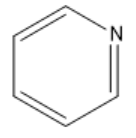
The lone pair on the nitrogen atom does not take place in delocalization and is not considered when counting the number of $ \pi $ electrons. Pyridine is an aromatic compound containing an amine. Aromatic compounds are considered very stable and they can only undergo reactions if the end product keeps the aromaticity of the ring. The aromatic compound pyridine has three resonance structures.
Therefore, pyridine is an aromatic compound.
Note :
The nitrogen present in pyridine creates a tertiary amine which is able to undergo alkylation and oxidation reactions. This amine causes a dipole on the ring, so it is not stable as other aromatic compounds like benzene. The dipole is the effect of more electrons being drawn towards the nitrogen atom instead of being shared equally with all the atoms present. This happens because of the fact that the nitrogen atom is more electronegative than the carbon atom.
Complete Step By Step Answer:
The $ \pi $ orbital system of pyridine has $ p $ electrons which are delocalized throughout the ring. It also has $ 4n + 2 $ delocalized $ p $ electrons, where $ n = 1 $ . Pyridine is planar as all of its $ p $ orbitals are perpendicular to the ring. Also, it is a cyclic compound. It matches all the qualifications of an aromatic compound. So, pyridine is an aromatic compound. The structure of pyridine is given below:

The lone pair on the nitrogen atom does not take place in delocalization and is not considered when counting the number of $ \pi $ electrons. Pyridine is an aromatic compound containing an amine. Aromatic compounds are considered very stable and they can only undergo reactions if the end product keeps the aromaticity of the ring. The aromatic compound pyridine has three resonance structures.
Therefore, pyridine is an aromatic compound.
Note :
The nitrogen present in pyridine creates a tertiary amine which is able to undergo alkylation and oxidation reactions. This amine causes a dipole on the ring, so it is not stable as other aromatic compounds like benzene. The dipole is the effect of more electrons being drawn towards the nitrogen atom instead of being shared equally with all the atoms present. This happens because of the fact that the nitrogen atom is more electronegative than the carbon atom.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




