
Is NH an electron withdrawing group?
Answer
513.3k+ views
Hint: Nitrogen has one lone pair of electrons. An electron withdrawing group is those which withdraw electrons from the central atom. In contrast, electron donating groups donate electron density to the central atom.
Complete answer:
In order to explain in more detail, let us consider the example of aniline. In aniline, nitrogen is \[s{{p}^{3}}\] hybridized. Two of the four \[s{{p}^{3}}\] hybrid orbitals form two covalent bonds with hydrogen atoms. Third \[s{{p}^{3}}\]hybrid orbital is bonded to adjacent carbon of benzene ring while fourth one contains a lone pair of electrons. This lone pair of electrons is delocalized over the benzene ring and then increases the electron density predominantly at ortho and para position. This is illustrated in the following diagram.
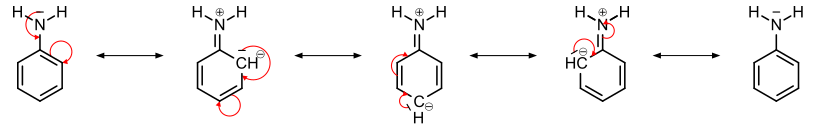
From the above diagram, we conclude that NH is an electron donating group instead of electron withdrawing. As NH group increases the electron density at ortho and para carbon of the benzene, hence it activates the benzene ring towards electrophilic substitution reaction. Therefore, NH acts as an activating group here. In contrast, when any moiety undergoes a nucleophilic substitution reaction then NH acts as a deactivating group.
Therefore, NH is an electron donating group instead of electron withdrawing.
Note:
It is important to note that NH is an electron donating group instead of electron withdrawing. Nitrogen has a lone pair of electrons which provide electron density to the central atom. Because of this NH group, aniline is more reactive in comparison to benzene towards electrophilic substitution reaction.
Complete answer:
In order to explain in more detail, let us consider the example of aniline. In aniline, nitrogen is \[s{{p}^{3}}\] hybridized. Two of the four \[s{{p}^{3}}\] hybrid orbitals form two covalent bonds with hydrogen atoms. Third \[s{{p}^{3}}\]hybrid orbital is bonded to adjacent carbon of benzene ring while fourth one contains a lone pair of electrons. This lone pair of electrons is delocalized over the benzene ring and then increases the electron density predominantly at ortho and para position. This is illustrated in the following diagram.

From the above diagram, we conclude that NH is an electron donating group instead of electron withdrawing. As NH group increases the electron density at ortho and para carbon of the benzene, hence it activates the benzene ring towards electrophilic substitution reaction. Therefore, NH acts as an activating group here. In contrast, when any moiety undergoes a nucleophilic substitution reaction then NH acts as a deactivating group.
Therefore, NH is an electron donating group instead of electron withdrawing.
Note:
It is important to note that NH is an electron donating group instead of electron withdrawing. Nitrogen has a lone pair of electrons which provide electron density to the central atom. Because of this NH group, aniline is more reactive in comparison to benzene towards electrophilic substitution reaction.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Explain zero factorial class 11 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

State and prove Bernoullis theorem class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE




