
Is ethene a planar molecule?
Answer
510k+ views
Hint: We have to say that ethene is IUPAC name of the compound ethylene. It is similar to the ethyl group but one double bond is found between two atoms of carbon. The chemical formula of ethene has ${C_2}{H_4}$ and it is the simplest alkene as it contains only two carbon atoms for carbon-carbon double bond.
Complete answer:
We can draw the structure of ethene is,
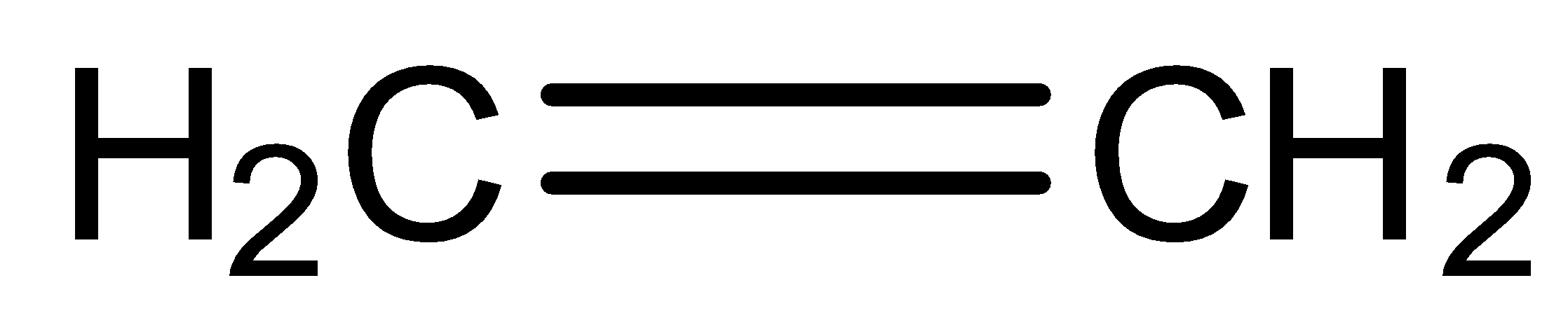
Ethene is comprised of four $1{s^1}$ hydrogen particles and two \[2{s^2}{\text{ }}2{p_x}^1{\text{ }}2{p_y}^2\] carbon molecules. These carbon particles as of now have four electrons, yet they each need to get four all the more so they have an entire eight in the valence shell. Having eight valence electrons around carbon gives the actual molecule a similar electron design as neon, an honorable gas. Carbon needs to have a similar setup as Neon since when it has eight valence electrons carbon is at its generally steady, least energy state, it has the entirety of the electrons that it needs, so it is not, at this point, receptive.
Ethene is anything but an extremely convoluted particle. It contains two carbon atoms that are double bonded attached to one another, with every one of these molecules likewise attached to two hydrogen atoms. This structures an aggregate of three bonds to every carbon particle, giving them $s{p^2}$hybridization. Since the carbon atom is shaping three sigma bonds rather than the four that it can, it just necessitates to hybridize three of its external orbitals, rather than four. It does this by utilizing the 2s electron and two of the 2p electrons, leaving the other unaltered. This new orbital is called a $s{p^2}$ half and half since that is actually what it will be, it is produced using one s orbital and two p orbitals. When molecules are a $s{p^2}$ hybrid they have a trigonal planar. These constructions are basically the same as a 'gesture of goodwill, there is a central atom with three molecules around it, all on one plane. Trigonal planar molecules have an ideal bond point of \[120^\circ \] on each side. The H-C-H bond point is \[117^\circ \], which is near the ideal \[120^\circ \] of a carbon with $s{p^2}$ hybridization. The other two points (H-C=C) are both \[121.5^\circ \].
Yes ethene is a planar molecule because there is no free rotation around the carbon-carbon double bond.
The molecule of ethene is a planar molecule.
Note:
We have to know that molecular geometry of an organic compound is based on the number of atoms that are attached to carbon. In ethene, three atoms are bonded to an atom of carbon which includes two atoms of hydrogen and one atom of carbon. So, the geometry of ethene is planar.
Complete answer:
We can draw the structure of ethene is,
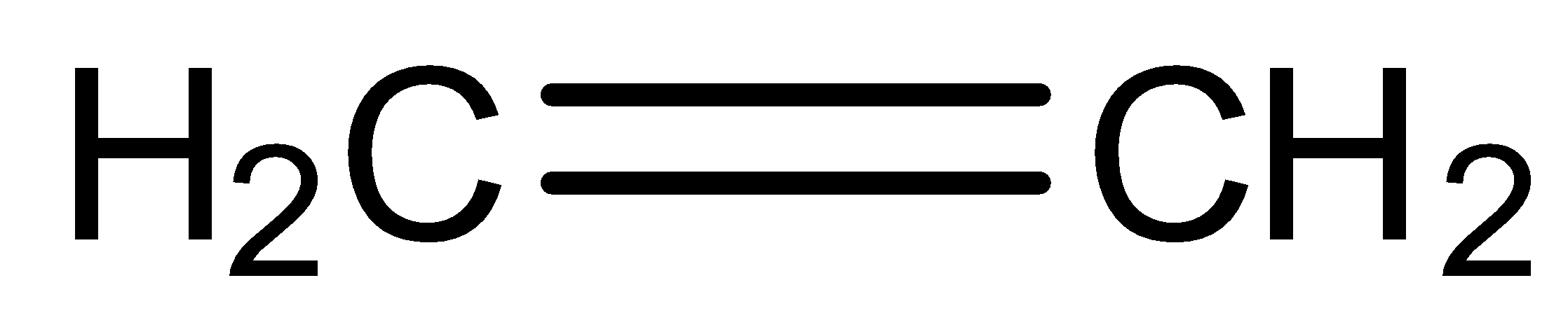
Ethene is comprised of four $1{s^1}$ hydrogen particles and two \[2{s^2}{\text{ }}2{p_x}^1{\text{ }}2{p_y}^2\] carbon molecules. These carbon particles as of now have four electrons, yet they each need to get four all the more so they have an entire eight in the valence shell. Having eight valence electrons around carbon gives the actual molecule a similar electron design as neon, an honorable gas. Carbon needs to have a similar setup as Neon since when it has eight valence electrons carbon is at its generally steady, least energy state, it has the entirety of the electrons that it needs, so it is not, at this point, receptive.
Ethene is anything but an extremely convoluted particle. It contains two carbon atoms that are double bonded attached to one another, with every one of these molecules likewise attached to two hydrogen atoms. This structures an aggregate of three bonds to every carbon particle, giving them $s{p^2}$hybridization. Since the carbon atom is shaping three sigma bonds rather than the four that it can, it just necessitates to hybridize three of its external orbitals, rather than four. It does this by utilizing the 2s electron and two of the 2p electrons, leaving the other unaltered. This new orbital is called a $s{p^2}$ half and half since that is actually what it will be, it is produced using one s orbital and two p orbitals. When molecules are a $s{p^2}$ hybrid they have a trigonal planar. These constructions are basically the same as a 'gesture of goodwill, there is a central atom with three molecules around it, all on one plane. Trigonal planar molecules have an ideal bond point of \[120^\circ \] on each side. The H-C-H bond point is \[117^\circ \], which is near the ideal \[120^\circ \] of a carbon with $s{p^2}$ hybridization. The other two points (H-C=C) are both \[121.5^\circ \].
Yes ethene is a planar molecule because there is no free rotation around the carbon-carbon double bond.
The molecule of ethene is a planar molecule.
Note:
We have to know that molecular geometry of an organic compound is based on the number of atoms that are attached to carbon. In ethene, three atoms are bonded to an atom of carbon which includes two atoms of hydrogen and one atom of carbon. So, the geometry of ethene is planar.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




