
Is $ C{O_2} $ polar or nonpolar?
Answer
489.9k+ views
Hint: To solve this question we should know about:
Polar molecules: A polar molecule is one that has a slightly positive one end and a slightly negative other end.
Non-Polar molecules: When the charge distribution of a molecule is averaged over time, it is spherically symmetric.
So, first we first try to draw the structure of given molecules then we try to correlate it with given molecules.
Complete Step By Step Answer:
As, $ C{O_2} $ Possesses two polar $ C - O $ bonds, but the geometry of $ C{O_2} $ is linear, therefore the two are not in direct contact. There is no net molecular dipole moment because bond dipole moments cancel.
The molecule is a nonpolar one.
Always remember:
Straight line = linear
Polarity is defined as the number of electrons that are not matched to the atom's major pain component. This can happen in a variety of elements, but it's most likely to happen in one with a positive or negative charge. When all of the electrons in a compound are matched to the elements in the complex, this occurs.
The most common associations with polarity are electrical charge and magnetism. As a result, if an element or compound is magnetic or conducts electricity well, it is said to have polarity.
When anything has polarity, you usually talk about di-poles, while mono-pole hypotheses are being researched in science. That simply implies that for di-poles (think of the North Pole-South Pole on the globe), there is a positive charge and a negative charge, but for mono-poles, the charges are still being defined.
Consider this: it is linear, with four dipoles from the oxygen atoms, yet the carbon atom can balance the polarity of those four dipoles. You wouldn't be able to run power via a chain of $ C{O_2} $ molecules. $ C{O_2} $ is also not magnetic.
We can understand it from the figure:
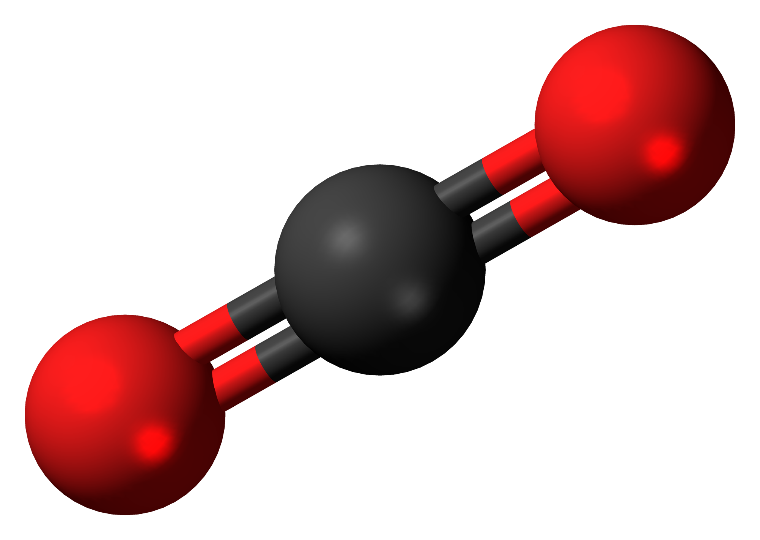
Note:
The relative electronegativity values between two atoms forming a chemical bond determine polarity. A covalent bond is formed when two atoms have the same electronegativity values. In a covalent link, electrons are shared evenly across atoms, making the bond nonpolar. Polar covalent bonds are formed by atoms with slightly differing electronegativity values. Ionic bonds arise when the electronegativity values of two atoms are very dissimilar. The polarity of ionic bonding is very high.
The polarity of the bonds and the polarity of the molecule are frequently the same. Nonpolar molecules with polar bonds do exist, as do polar molecules with nonpolar bonds! Boron trifluoride, for example, is a nonpolar compound with polar covalent connections.
Polar molecules: A polar molecule is one that has a slightly positive one end and a slightly negative other end.
Non-Polar molecules: When the charge distribution of a molecule is averaged over time, it is spherically symmetric.
So, first we first try to draw the structure of given molecules then we try to correlate it with given molecules.
Complete Step By Step Answer:
As, $ C{O_2} $ Possesses two polar $ C - O $ bonds, but the geometry of $ C{O_2} $ is linear, therefore the two are not in direct contact. There is no net molecular dipole moment because bond dipole moments cancel.
The molecule is a nonpolar one.
Always remember:
Straight line = linear
Polarity is defined as the number of electrons that are not matched to the atom's major pain component. This can happen in a variety of elements, but it's most likely to happen in one with a positive or negative charge. When all of the electrons in a compound are matched to the elements in the complex, this occurs.
The most common associations with polarity are electrical charge and magnetism. As a result, if an element or compound is magnetic or conducts electricity well, it is said to have polarity.
When anything has polarity, you usually talk about di-poles, while mono-pole hypotheses are being researched in science. That simply implies that for di-poles (think of the North Pole-South Pole on the globe), there is a positive charge and a negative charge, but for mono-poles, the charges are still being defined.
Consider this: it is linear, with four dipoles from the oxygen atoms, yet the carbon atom can balance the polarity of those four dipoles. You wouldn't be able to run power via a chain of $ C{O_2} $ molecules. $ C{O_2} $ is also not magnetic.
We can understand it from the figure:

Note:
The relative electronegativity values between two atoms forming a chemical bond determine polarity. A covalent bond is formed when two atoms have the same electronegativity values. In a covalent link, electrons are shared evenly across atoms, making the bond nonpolar. Polar covalent bonds are formed by atoms with slightly differing electronegativity values. Ionic bonds arise when the electronegativity values of two atoms are very dissimilar. The polarity of ionic bonding is very high.
The polarity of the bonds and the polarity of the molecule are frequently the same. Nonpolar molecules with polar bonds do exist, as do polar molecules with nonpolar bonds! Boron trifluoride, for example, is a nonpolar compound with polar covalent connections.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




