
Is \[CC{l_4}\] polar or nonpolar?
Answer
510.3k+ views
Hint : The tetrahedral arrangement of chlorines around the central carbon gives rise to a symmetrical spatial arrangement which decides the polarity of carbon tetrachloride as electronegativity difference alone cannot be used to distinguish between a polar or nonpolar molecule.
Complete Step By Step Answer:
Carbon tetrachloride is an organic compound that can be formed by the halogenation reaction between methane and chlorine in the presence of sunlight.
The chlorines are arranged in a tetrahedral fashion around the carbon atom leading to a symmetrical arrangement.
Polarity of a bond is a consequence of partial charge separation in covalent bonds due to the electronegativity differences.
Chlorine being a halogen has a high effective nuclear charge that leads to high electronegativity that renders the carbon atom slightly electropositive. This electronegativity difference between carbon and chlorine makes their bond polar.
But bond polarity solely cannot determine the nature of the entire compound. As four equidistant bonds are arranged symmetrically where the dipole moment is directed towards the more electronegative chlorine atoms. The dipole moment being a vector gets cancelled when the magnitude is same and the directions are opposite.
The dipole moment of one bond cancels that of another placed opposite to it. Hence the two pairs of bonds in carbon tetrachloride cancel each other resulting in net zero dipole moment.
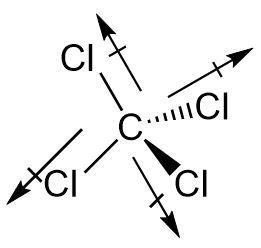
Therefore carbon tetrachloride \[CC{l_4}\] is a nonpolar molecule.
Note :
A different structure of carbon tetrachloride could have resulted in a polar molecule as the bonds themselves are polar. A pyramidal geometry like that of ammonia where three bonds face one direction while one bond faces the other would result in some net dipole moment.
Complete Step By Step Answer:
Carbon tetrachloride is an organic compound that can be formed by the halogenation reaction between methane and chlorine in the presence of sunlight.
The chlorines are arranged in a tetrahedral fashion around the carbon atom leading to a symmetrical arrangement.
Polarity of a bond is a consequence of partial charge separation in covalent bonds due to the electronegativity differences.
Chlorine being a halogen has a high effective nuclear charge that leads to high electronegativity that renders the carbon atom slightly electropositive. This electronegativity difference between carbon and chlorine makes their bond polar.
But bond polarity solely cannot determine the nature of the entire compound. As four equidistant bonds are arranged symmetrically where the dipole moment is directed towards the more electronegative chlorine atoms. The dipole moment being a vector gets cancelled when the magnitude is same and the directions are opposite.
The dipole moment of one bond cancels that of another placed opposite to it. Hence the two pairs of bonds in carbon tetrachloride cancel each other resulting in net zero dipole moment.

Therefore carbon tetrachloride \[CC{l_4}\] is a nonpolar molecule.
Note :
A different structure of carbon tetrachloride could have resulted in a polar molecule as the bonds themselves are polar. A pyramidal geometry like that of ammonia where three bonds face one direction while one bond faces the other would result in some net dipole moment.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




