
Iron carbonyl, \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\] is:
A. Trinuclear
B. Mononuclear
C. Tetranuclear
D. Dinuclear
Answer
620.4k+ views
Hint: Here, we will proceed by defining the nuclearity of the coordinate compounds. Then draw the structural formula of Iron carbonyl \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\] to find the nuclearity of the given coordinate compound.
Complete answer:
Definition of Nuclearity: The nuclearity of a single coordination entity indicates the number of central atoms joined by bridging ligands or metal-metal bonds.
The simplest nuclearity is mononuclear, followed by dinuclear, trinuclear, tetranuclear, pentanuclear…………………………., polynuclear.
Iron pentacarbonyl, also known as Iron carbonyl, is the compound with compound with formula \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\].
The structural formula of Iron carbonyl \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\] is:
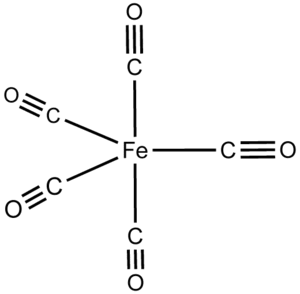
From the structural form of Iron carbonyl \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\], one Fe atom is surrounded by 5 CO ligands.
So, Iron carbonyl \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\] is mononuclear.
Note: Under standard conditions \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\] is a free-flowing, straw-coloured liquid with a pungent smell. Mononuclear complexes are the simplest types of coordination compounds that contain a single metal atom or ion surrounded by monodentate ligands.
Complete answer:
Definition of Nuclearity: The nuclearity of a single coordination entity indicates the number of central atoms joined by bridging ligands or metal-metal bonds.
The simplest nuclearity is mononuclear, followed by dinuclear, trinuclear, tetranuclear, pentanuclear…………………………., polynuclear.
Iron pentacarbonyl, also known as Iron carbonyl, is the compound with compound with formula \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\].
The structural formula of Iron carbonyl \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\] is:

From the structural form of Iron carbonyl \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\], one Fe atom is surrounded by 5 CO ligands.
So, Iron carbonyl \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\] is mononuclear.
Note: Under standard conditions \[{\text{Fe}}{\left( {{\text{CO}}} \right)_5}\] is a free-flowing, straw-coloured liquid with a pungent smell. Mononuclear complexes are the simplest types of coordination compounds that contain a single metal atom or ion surrounded by monodentate ligands.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Organisms of a higher trophic level which feed on several class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE




