
Intramolecular hydrogen bonding is found in:
A. salicylaldehyde
B. water
C. acetaldehyde
D. phenol
Answer
536.4k+ views
Hint: Hydrogen bonding is a dipole-dipole attraction between the molecules, but is not a covalent bond to a hydrogen atom. When the hydrogen is attached to a very electronegative element, then this type of bonding takes place. It is represented by a dotted line.
Complete step by step answer:
Hydrogen bond or H-bond is a partial intermolecular bond between a lone pair which is on the electron rich donor atom and the antibonding molecular orbital of a H atom. Hydrogen bonds are of two types: intramolecular hydrogen bond and intermolecular hydrogen bond. In intramolecular H-bonding, the bonds occur in the same molecule of a solution whereas, in intermolecular H-bonding, the bonds occur between two different molecules of a solution.
Now, let's look at the options.
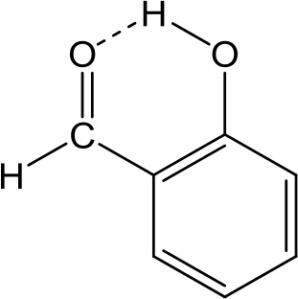
A. In salicylaldehyde, intramolecular H-bonds exist between the hydrogen atom from the hydroxy group and the oxygen atom from the aldehyde group.
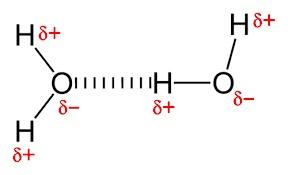
B. In water, the intermolecular H-bonding exists between the hydrogen atom from one molecule and the oxygen atom from another molecule.
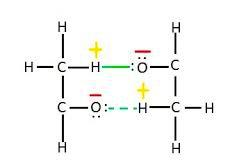
C. In acetaldehyde, the intermolecular H-bonding exists between the hydrogen atom from one molecule and the oxygen atom from another molecule.
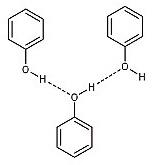
D. In phenol, the intermolecular H-bonding exists between the hydrogen atom from one molecule and the oxygen atom from another molecule.
Therefore, from the above diagrams, we can see that only salicylic acid has intramolecular hydrogen bonding.
Hence, the correct answer is option (a).
Note: Salicylic acid is a strong acid. The hydrogen bonding in salicylic acid lowers its free energy which makes salicylate a weaker conjugate base. And thus, salicylic acid is a strong acid.
Complete step by step answer:
Hydrogen bond or H-bond is a partial intermolecular bond between a lone pair which is on the electron rich donor atom and the antibonding molecular orbital of a H atom. Hydrogen bonds are of two types: intramolecular hydrogen bond and intermolecular hydrogen bond. In intramolecular H-bonding, the bonds occur in the same molecule of a solution whereas, in intermolecular H-bonding, the bonds occur between two different molecules of a solution.
Now, let's look at the options.
A. In salicylaldehyde, intramolecular H-bonds exist between the hydrogen atom from the hydroxy group and the oxygen atom from the aldehyde group.

B. In water, the intermolecular H-bonding exists between the hydrogen atom from one molecule and the oxygen atom from another molecule.

C. In acetaldehyde, the intermolecular H-bonding exists between the hydrogen atom from one molecule and the oxygen atom from another molecule.

D. In phenol, the intermolecular H-bonding exists between the hydrogen atom from one molecule and the oxygen atom from another molecule.

Therefore, from the above diagrams, we can see that only salicylic acid has intramolecular hydrogen bonding.
Hence, the correct answer is option (a).
Note: Salicylic acid is a strong acid. The hydrogen bonding in salicylic acid lowers its free energy which makes salicylate a weaker conjugate base. And thus, salicylic acid is a strong acid.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




