
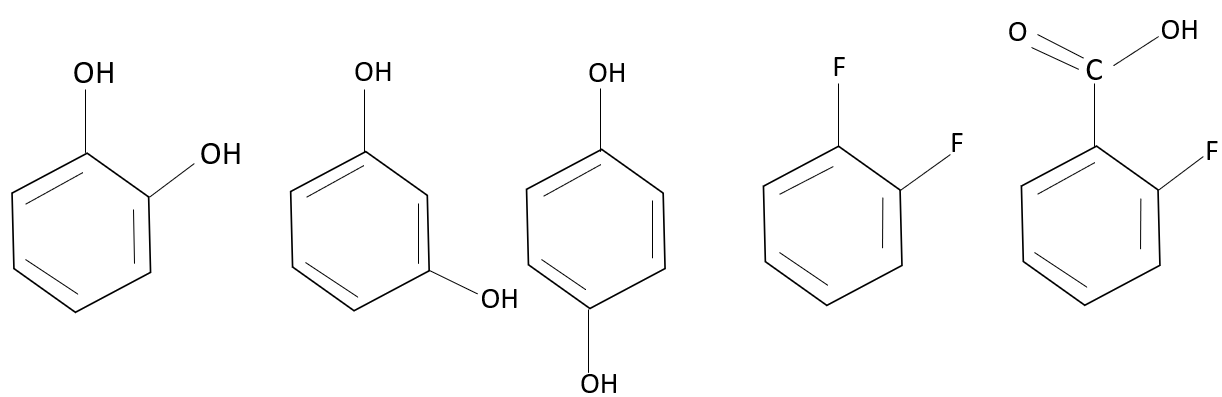
Intermolecular H-Bonding is observed in how many of the following?

Answer
571.8k+ views
Hint:Intermolecular bonds are formed by the attraction between the negative part of one molecule and the positive part of another molecule. The intermolecular bond is stronger than the intramolecular bond.
Complete step by step answer:
The intermolecular bond takes place between two molecules, one containing hydrogen and the other containing electronegative atoms such as oxygen, nitrogen, or fluorine. Here, the compound catechol has two hydroxyl groups, they are attached at ortho position; hence, they undergo intramolecular force of attraction (bonding within a molecule), and similarly with resorcinol the presence of two hydroxyl groups at meta position, it undergoes bonding between a hydrogen atom and the oxygen atom of the same molecule, thereby undergoing intramolecular bonding. Whereas, the para hydroxy phenol undergoes intermolecular bonding, as they cannot undergo an intramolecular bond. The presence of the hydroxyl group at the para position undergoes intermolecular bonding with the other molecule. The compound 1,2 difluoro benzene undergoes intermolecular force of bonding since there is no interaction or presence of hydrogen within the compound to undergo intramolecular bonding; hence, the fluorine molecule undergoes intermolecular hydrogen bonding with another molecule. The 2-fluoro benzoic acid undergoes intramolecular bonding. The hydrogen of the carboxylic group undergoes bonding with the fluorine in the second position.
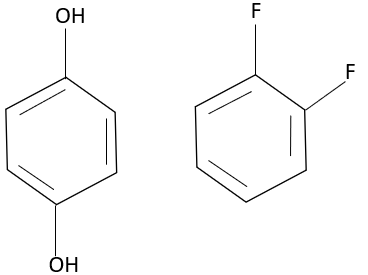
Thus, para hydroxy phenol and 1,2 difluoro benzene undergo intermolecular bonding.
Note:
Hydrogen bonding is said to be a secondary type of bonding, it is a weaker bond. The hydrogen bonding is synonymous with Van Der Waals force of attraction. The hydrogen bond is responsible for the existence of solids of many organic molecules containing hydroxyl groups such as glucose and sucrose.
Complete step by step answer:
The intermolecular bond takes place between two molecules, one containing hydrogen and the other containing electronegative atoms such as oxygen, nitrogen, or fluorine. Here, the compound catechol has two hydroxyl groups, they are attached at ortho position; hence, they undergo intramolecular force of attraction (bonding within a molecule), and similarly with resorcinol the presence of two hydroxyl groups at meta position, it undergoes bonding between a hydrogen atom and the oxygen atom of the same molecule, thereby undergoing intramolecular bonding. Whereas, the para hydroxy phenol undergoes intermolecular bonding, as they cannot undergo an intramolecular bond. The presence of the hydroxyl group at the para position undergoes intermolecular bonding with the other molecule. The compound 1,2 difluoro benzene undergoes intermolecular force of bonding since there is no interaction or presence of hydrogen within the compound to undergo intramolecular bonding; hence, the fluorine molecule undergoes intermolecular hydrogen bonding with another molecule. The 2-fluoro benzoic acid undergoes intramolecular bonding. The hydrogen of the carboxylic group undergoes bonding with the fluorine in the second position.
Thus, para hydroxy phenol and 1,2 difluoro benzene undergo intermolecular bonding.

Note:
Hydrogen bonding is said to be a secondary type of bonding, it is a weaker bond. The hydrogen bonding is synonymous with Van Der Waals force of attraction. The hydrogen bond is responsible for the existence of solids of many organic molecules containing hydroxyl groups such as glucose and sucrose.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




