
What is the instantaneous rate of reaction? And how can we determine it?
Answer
564.3k+ views
Hint:The term instantaneous rate means the reaction rate at a particular time of very short interval. We determine it with the help of plotting the reaction rate vs. time.
Complete step by step answer:
The instantaneous rate of reaction is the rate at some instant at a particular time. Specifically it is defined as the change in concentration of the components of a reaction at an infinitely small time interval. The average of the instantaneous reaction rate over a period of time gives the average rate during the reaction.
For determining the instantaneous rate at a definite time t we need to plot the change in rate of the rate of reaction along the x axis and the time interval along the y axis. Using the plot we calculate the negative of the slope of the curve of the change in concentration of a reactant with respect to the time at time \[t\]. It is done by measuring the slope of the tangent to the graph of concentration vs. time.
Let us consider a reaction in which a reactant \[R\] is converting into a product \[P\]. The reaction is
$R \to P$
If we plot the change in the concentration of the formation of \[P\]with time \[t\], we obtain a curved line instead of a straight line. The plot is as follows:
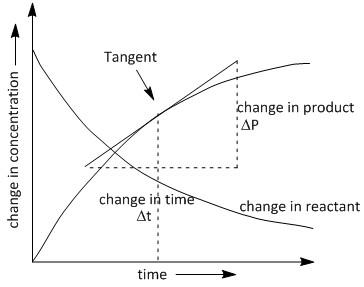
In the above graph we need to draw the best tangent at the time \[t\]where we require the rate of reaction. By extending the endpoints of the tangent along the x and y axis we obtain a triangle where it is convenient to calculate the value of change in concentration of product P with time i.e. $\dfrac{{\Delta P}}{{\Delta t}}$ .
The instantaneous rate of reaction is determined using the slope of the line or the tangent to the curve at any time (\[t\]).
Note:
The rate of concentration of products increases with time according to the plot seen above and the rate of concentration of reactants decreases with time. Thus a negative slope is obtained in plotting the change in concentration of reactant with time.
Complete step by step answer:
The instantaneous rate of reaction is the rate at some instant at a particular time. Specifically it is defined as the change in concentration of the components of a reaction at an infinitely small time interval. The average of the instantaneous reaction rate over a period of time gives the average rate during the reaction.
For determining the instantaneous rate at a definite time t we need to plot the change in rate of the rate of reaction along the x axis and the time interval along the y axis. Using the plot we calculate the negative of the slope of the curve of the change in concentration of a reactant with respect to the time at time \[t\]. It is done by measuring the slope of the tangent to the graph of concentration vs. time.
Let us consider a reaction in which a reactant \[R\] is converting into a product \[P\]. The reaction is
$R \to P$
If we plot the change in the concentration of the formation of \[P\]with time \[t\], we obtain a curved line instead of a straight line. The plot is as follows:

In the above graph we need to draw the best tangent at the time \[t\]where we require the rate of reaction. By extending the endpoints of the tangent along the x and y axis we obtain a triangle where it is convenient to calculate the value of change in concentration of product P with time i.e. $\dfrac{{\Delta P}}{{\Delta t}}$ .
The instantaneous rate of reaction is determined using the slope of the line or the tangent to the curve at any time (\[t\]).
Note:
The rate of concentration of products increases with time according to the plot seen above and the rate of concentration of reactants decreases with time. Thus a negative slope is obtained in plotting the change in concentration of reactant with time.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




