
In Williamson's synthesis, ethoxy ethane is prepared by,
A.Heating sodium ethoxide with ethyl bromide
B.Passing ethanol over heated alumina
C.Treating ethyl alcohol with excess of conc. ${{H}_{2}}S{{O}_{4}}$ at 430 – 440 K
D.Heating ethanol with dry $A{{g}_{2}}O$
Answer
591.6k+ views
Hint: We know that Williamson's synthesis is one of the best methods for the preparation of both simple and mixed ethers. It’s a coupling reaction. The reaction was developed by Alexander William Williamson.
Complete answer:
We know that Williamson's synthesis is the reaction of alkyl halides with sodium alkoxide or sodium phenoxide to form ethers.
Let’s now see the chemical reaction in Williamson’s synthesis .
For example, For example, $R-X+R'-ONa\xrightarrow{\vartriangle }R-O-R'+NaX$
The Alkyl halide reacts with the sodium alkoxide in presence of heat to produce Ether and sodium halide as a byproduct.
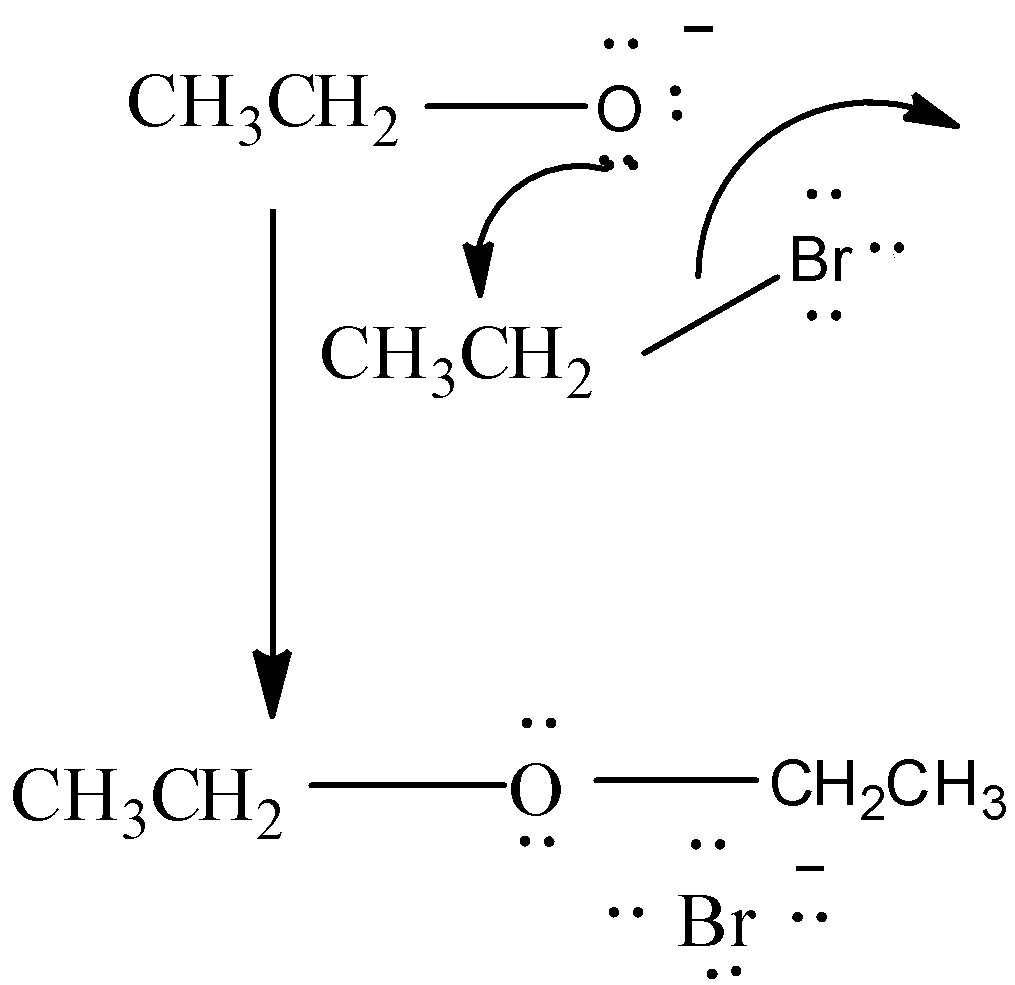
$C{{H}_{3}}C{{H}_{2}}Br+C{{H}_{3}}C{{H}_{2}}ONa\xrightarrow{\vartriangle }C{{H}_{3}}C{{H}_{2}}-O-C{{H}_{2}}C{{H}_{3}}+NaBr$
At first, we get Sodium ethoxide ( $C{{H}_{3}}C{{H}_{2}}{{O}^{-}}N{{a}^{+}}$) by passing metallic $Na$ through Ethanol $(C{{H}_{3}}C{{H}_{2}}OH)$ . Then, Ethyl Bromide reacts with Sodium ethoxide to form Ethoxy ethane (IUPAC Name: Diethyl ether) via${{S}_{N}}^{2}$ reaction.
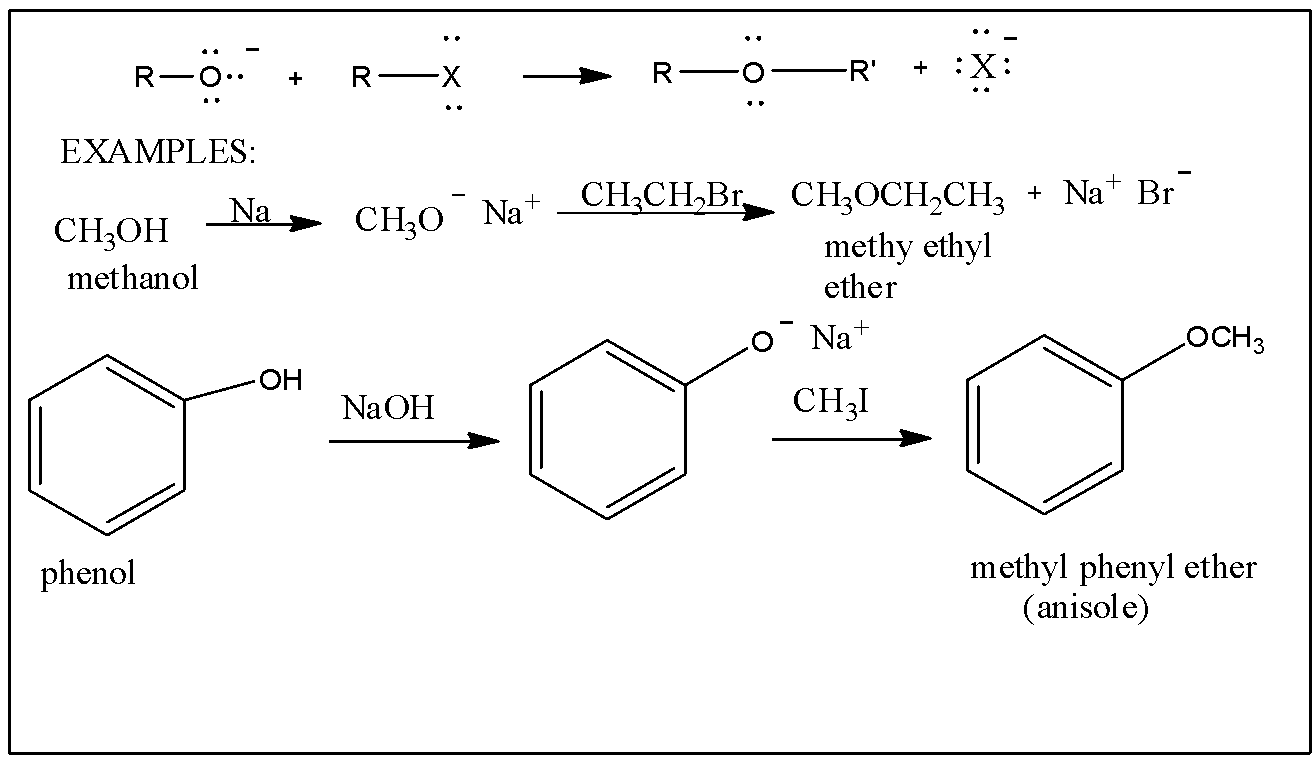
The correct option is A. Heating Sodium ethoxide with Ethyl bromide.
Note: Williamson’s synthesis is only possible in case of primary alkyl halide used in the chemical Williamson’s synthesis is only possible in case of primary alkyl halide used in the chemical reaction. If higher degree of alkyl halide is used i.e. Secondary alkyl halide and Tertiary alkyl halide then, the reaction will go through $E2$ type of reaction instead of ${{S}_{N}}^{2}$ Reaction and result in the formation of a Alkene in the product section.
Complete answer:
We know that Williamson's synthesis is the reaction of alkyl halides with sodium alkoxide or sodium phenoxide to form ethers.
Let’s now see the chemical reaction in Williamson’s synthesis .
For example, For example, $R-X+R'-ONa\xrightarrow{\vartriangle }R-O-R'+NaX$
The Alkyl halide reacts with the sodium alkoxide in presence of heat to produce Ether and sodium halide as a byproduct.

$C{{H}_{3}}C{{H}_{2}}Br+C{{H}_{3}}C{{H}_{2}}ONa\xrightarrow{\vartriangle }C{{H}_{3}}C{{H}_{2}}-O-C{{H}_{2}}C{{H}_{3}}+NaBr$
At first, we get Sodium ethoxide ( $C{{H}_{3}}C{{H}_{2}}{{O}^{-}}N{{a}^{+}}$) by passing metallic $Na$ through Ethanol $(C{{H}_{3}}C{{H}_{2}}OH)$ . Then, Ethyl Bromide reacts with Sodium ethoxide to form Ethoxy ethane (IUPAC Name: Diethyl ether) via${{S}_{N}}^{2}$ reaction.

The correct option is A. Heating Sodium ethoxide with Ethyl bromide.
Note: Williamson’s synthesis is only possible in case of primary alkyl halide used in the chemical Williamson’s synthesis is only possible in case of primary alkyl halide used in the chemical reaction. If higher degree of alkyl halide is used i.e. Secondary alkyl halide and Tertiary alkyl halide then, the reaction will go through $E2$ type of reaction instead of ${{S}_{N}}^{2}$ Reaction and result in the formation of a Alkene in the product section.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




