
In which of the following liquid pairs, there is contraction in volume on mixing?
A. $ CHC{l_3} + {C_6}{H_6} $
B. $ {H_2}O + HCl $
C. $ {H_2}O + HN{O_3} $
D. $ {H_2}O + {C_2}{H_5}OH $
Answer
521.1k+ views
Hint : Raoult’s law- It expresses a relationship between mole fraction and partial pressure of the volatile liquids. According to this law, the mole fraction of a solute in the solution is directly proportional to its partial pressure. The solutions are categorized into two categories on the basis of Raoult’s law i.e., ideal solution and non-ideal solution.
Complete Step By Step Answer:
When after mixing the solution, change in enthalpy is not equal to zero or expansion or contraction in volume of the mixture is observed, then it is said to be a non-ideal solution. Two types of deviations are observed in non-ideal solutions which are as follows:
Case-1: When expansion of the volume of mixture takes place, then it is said to be a positive deviation. In this condition, the intermolecular force of attraction of mixture is weaker than the original liquids i.e., solute-solvent forces are weaker than solute-solute and solvent-solvent interactions, therefore on liquids require to absorb heat to form a mixture i.e., it is an endothermic process.
Change in enthalpy of the mixture is greater than zero i.e., $ {\Delta _{mix}}H > 0 $ and expansion in volume of mixture is observed i.e., $ {\Delta _{mix}}V > 0 $ .
Examples of positive deviation are as follows:
1. $ {H_2}O + {C_2}{H_5}OH $
2. acetone and ethanol
Graphically, positive deviation in solutions is shown as follows:

Case-2: When the contraction of the volume of mixture takes place, then it is said to be a negative deviation. In this condition, the intermolecular force of attraction of mixture is stronger than the original liquids i.e., solute-solvent forces are stronger than solute-solute and solvent-solvent interactions, therefore on liquids release heat to form a mixture i.e., it is an exothermic process.
Change in enthalpy of the mixture is less than zero i.e., $ {\Delta _{mix}}H < 0 $ and contraction in volume of mixture is observed i.e., $ {\Delta _{mix}}V < 0 $ .
Examples of negative deviation are as follows:
1. $ CHC{l_3} + {C_6}{H_6} $
2. $ {H_2}O + HCl $
3. $ {H_2}O + HN{O_3} $
Graphically, negative deviation in solutions is shown as follows:
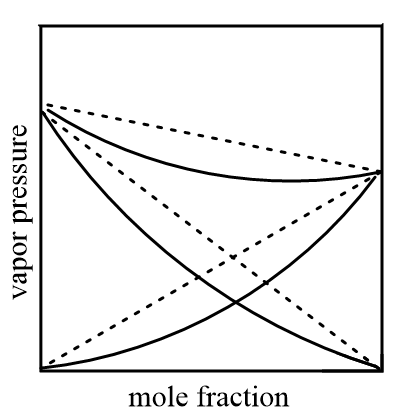
Hence, options (A), (B) and (C) are the correct answers.
Note :
It is important to note that in case of ideal solutions, the change in enthalpy of mixing of solutions is zero i.e., $ {\Delta _{mix}}H = 0 $ and the total volume of the mixture must be equal to the summation of volume of solute and solvents i.e., $ {\Delta _{mix}}V = 0 $ . Examples of the ideal solution are benzene and toluene, chlorobenzene and bromobenzene, etc.
Complete Step By Step Answer:
When after mixing the solution, change in enthalpy is not equal to zero or expansion or contraction in volume of the mixture is observed, then it is said to be a non-ideal solution. Two types of deviations are observed in non-ideal solutions which are as follows:
Case-1: When expansion of the volume of mixture takes place, then it is said to be a positive deviation. In this condition, the intermolecular force of attraction of mixture is weaker than the original liquids i.e., solute-solvent forces are weaker than solute-solute and solvent-solvent interactions, therefore on liquids require to absorb heat to form a mixture i.e., it is an endothermic process.
Change in enthalpy of the mixture is greater than zero i.e., $ {\Delta _{mix}}H > 0 $ and expansion in volume of mixture is observed i.e., $ {\Delta _{mix}}V > 0 $ .
Examples of positive deviation are as follows:
1. $ {H_2}O + {C_2}{H_5}OH $
2. acetone and ethanol
Graphically, positive deviation in solutions is shown as follows:

Case-2: When the contraction of the volume of mixture takes place, then it is said to be a negative deviation. In this condition, the intermolecular force of attraction of mixture is stronger than the original liquids i.e., solute-solvent forces are stronger than solute-solute and solvent-solvent interactions, therefore on liquids release heat to form a mixture i.e., it is an exothermic process.
Change in enthalpy of the mixture is less than zero i.e., $ {\Delta _{mix}}H < 0 $ and contraction in volume of mixture is observed i.e., $ {\Delta _{mix}}V < 0 $ .
Examples of negative deviation are as follows:
1. $ CHC{l_3} + {C_6}{H_6} $
2. $ {H_2}O + HCl $
3. $ {H_2}O + HN{O_3} $
Graphically, negative deviation in solutions is shown as follows:

Hence, options (A), (B) and (C) are the correct answers.
Note :
It is important to note that in case of ideal solutions, the change in enthalpy of mixing of solutions is zero i.e., $ {\Delta _{mix}}H = 0 $ and the total volume of the mixture must be equal to the summation of volume of solute and solvents i.e., $ {\Delta _{mix}}V = 0 $ . Examples of the ideal solution are benzene and toluene, chlorobenzene and bromobenzene, etc.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




