
In the soap micelles:
A.The ionic end of the soap is on the surface of the cluster while the carbon chain is in the interior of the cluster
B.The ionic end of the soap is in the interior of the cluster and the carbon chain is out of the cluster
C.Both the ionic and carbon chain are in the interior of the cluster
D.Both the ionic and carbon chain are in the exterior of the cluster
Answer
589.5k+ views
Hint: In soap micelles represent tiny hydrophobic pockets floating around in water, and can solubilize other hydrophobic molecules, such as oils etc. This is usually how a soap aids in cleaning.
Complete step by step answer:
To understand this question, let us first understand a few concepts:
The chemical structure of a soap consists of a polar ionic hydrophilic end (which basically means the end which is attracted to water) and a non – polar hydrophobic end (which basically means the end which repels water).
When this soap molecule is interacting with water, the soap molecules arrange themselves in the form of spherical aggregates called ‘micelles.’
These micelles have their hydrophilic ends on the outside, which get solvated by the water molecules. On the other hand, the hydrophobic end of the micelle gets clustered together away from the water.
The hydrophilic part of the micelle attaches to water while the hydrophobic part attaches itself to fibre and to dirt particle and completes the cleaning action of the soap
Now, the polar hydrophilic part of the micelle is ionic in nature, while the non – polar hydrophobic part is a carbon chain. The structure of a soap molecule can be given as:
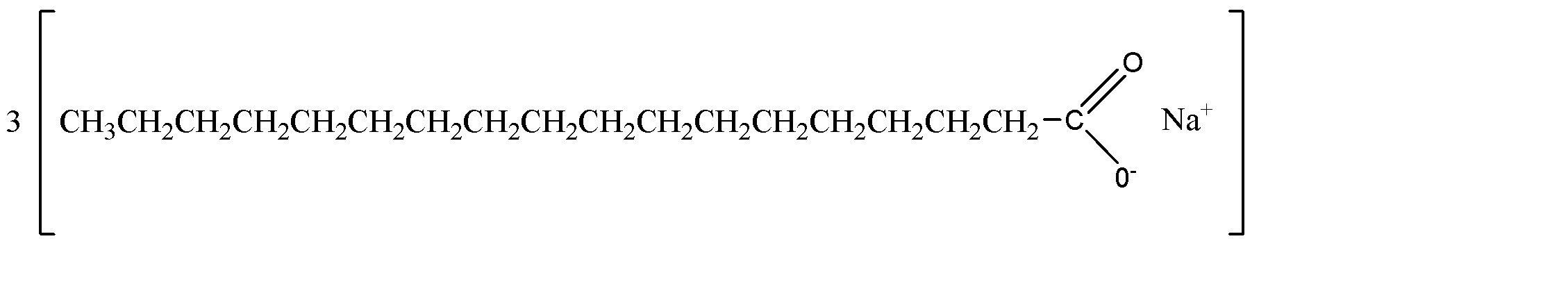
Hence from the above explanations, we can conclude that in the soap micelles the ionic end of the soap is on the surface of the cluster while the carbon chain is in the interior of the cluster.
Hence, Option A is the correct option.
Note:
Hydrolysis of a fat under basic conditions, such as with hydroxide, generates glycerol and three molecules of the fatty acid carboxylate. It is these carboxylates that are the soap molecules, and historically this is exactly how soap was made.
Complete step by step answer:
To understand this question, let us first understand a few concepts:
The chemical structure of a soap consists of a polar ionic hydrophilic end (which basically means the end which is attracted to water) and a non – polar hydrophobic end (which basically means the end which repels water).
When this soap molecule is interacting with water, the soap molecules arrange themselves in the form of spherical aggregates called ‘micelles.’
These micelles have their hydrophilic ends on the outside, which get solvated by the water molecules. On the other hand, the hydrophobic end of the micelle gets clustered together away from the water.
The hydrophilic part of the micelle attaches to water while the hydrophobic part attaches itself to fibre and to dirt particle and completes the cleaning action of the soap
Now, the polar hydrophilic part of the micelle is ionic in nature, while the non – polar hydrophobic part is a carbon chain. The structure of a soap molecule can be given as:
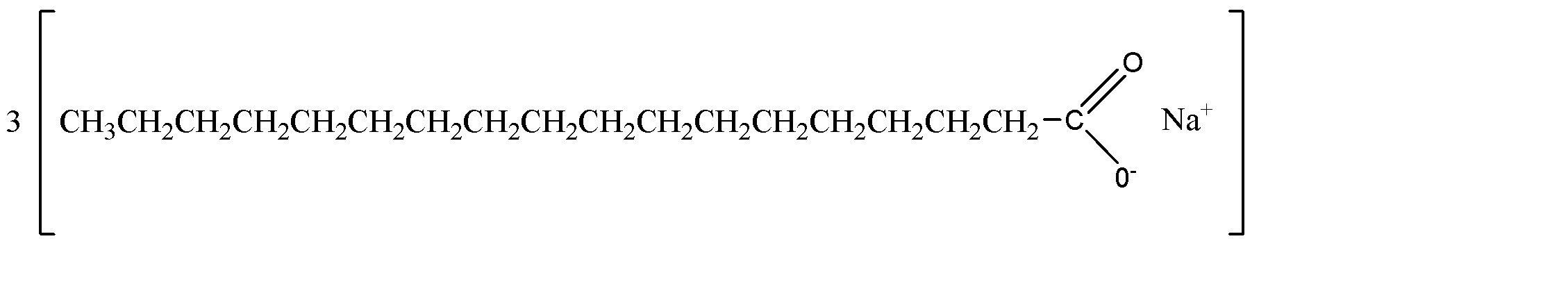
Hence from the above explanations, we can conclude that in the soap micelles the ionic end of the soap is on the surface of the cluster while the carbon chain is in the interior of the cluster.
Hence, Option A is the correct option.
Note:
Hydrolysis of a fat under basic conditions, such as with hydroxide, generates glycerol and three molecules of the fatty acid carboxylate. It is these carboxylates that are the soap molecules, and historically this is exactly how soap was made.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




