
In the scheme given above, the total number of intramolecular aldol condensation products formed from Y are:
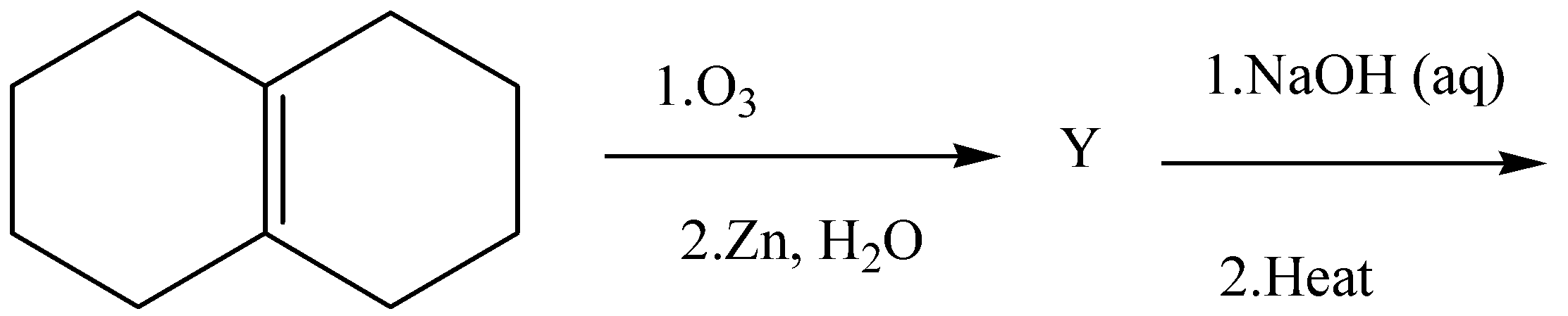
A.0
B.1
C.2
D.3
E.4
F.5
G.6
H.7
I.8
J.9
Answer
590.1k+ views
Hint: We must remember that the Ozonolysis is an organic reaction in which the unsaturated double or triple bonds of alkenes or alkynes are cleaved by the use of ozone. The alkenes or alkynes form organic compounds in which carbon-carbon bonds are replaced by the carbonyl group.
Complete step by step answer:We must remember that the oxygen allotrope is known as ozone. In this question first ozonolysis is happening by cleaving a carbon-carbon sigma bond. So let’s discuss a little about ozonolysis before moving forward. Ozonolysis is an organic reaction in which the unsaturated double or triple bonds of alkenes or alkynes are cleaved by the use of ozone. The alkenes or alkynes form organic compounds in which carbon-carbon bonds are replaced by the carbonyl group.
In this question the compound given to us is 1,2,3,4,5,6,7,8-octahydronaphthalene which undergoes ozonolysis which is followed by the reduction of this compound to cyclodecane-1,6-dione. After this the product Y which is cyclodecane-1,6-dione undergoes the Intramolecular aldol condensation produces only one aldol which is octahydro azulen-4(5H)-one.
Hence the answer of this question is option B.
Note:We also know that ozonolysis is a reaction which is used for the preparation of many industry based pharmaceuticals, along with used in researches. It is also used in natural synthesis reactions. By ozonolysis the cleavage of unsaturation happens which in turn gives us ketone compounds. We must remember that the ozonolysis of alkenes gives carboxylic acid, alcohols, aldehydes or ketones. Ozonolysis of alkynes gives diketones or acid anhydrides.
Complete step by step answer:We must remember that the oxygen allotrope is known as ozone. In this question first ozonolysis is happening by cleaving a carbon-carbon sigma bond. So let’s discuss a little about ozonolysis before moving forward. Ozonolysis is an organic reaction in which the unsaturated double or triple bonds of alkenes or alkynes are cleaved by the use of ozone. The alkenes or alkynes form organic compounds in which carbon-carbon bonds are replaced by the carbonyl group.
In this question the compound given to us is 1,2,3,4,5,6,7,8-octahydronaphthalene which undergoes ozonolysis which is followed by the reduction of this compound to cyclodecane-1,6-dione. After this the product Y which is cyclodecane-1,6-dione undergoes the Intramolecular aldol condensation produces only one aldol which is octahydro azulen-4(5H)-one.
Hence the answer of this question is option B.
Note:We also know that ozonolysis is a reaction which is used for the preparation of many industry based pharmaceuticals, along with used in researches. It is also used in natural synthesis reactions. By ozonolysis the cleavage of unsaturation happens which in turn gives us ketone compounds. We must remember that the ozonolysis of alkenes gives carboxylic acid, alcohols, aldehydes or ketones. Ozonolysis of alkynes gives diketones or acid anhydrides.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




