
In the Reimer Tiemann reaction dichlorocarbene acts as:
A. Nucleophile
B. Electrophile
C. Free radical
D. All of these
Answer
576.9k+ views
Hint: To solve this question one should know the complete Reimer Tiemann reaction. One should know the reactants used in the reaction and the products formed under the favorable conditions used in the reactions. We should know the proper mechanism of the reaction otherwise we will not be able to conclude about the dichlorocarbene. First, we will understand the conceptual meaning of the Reimer Tiemann reaction with the proper mechanism, and with the help of the reaction mechanism, we will be able to conclude about dichlorocarbene.
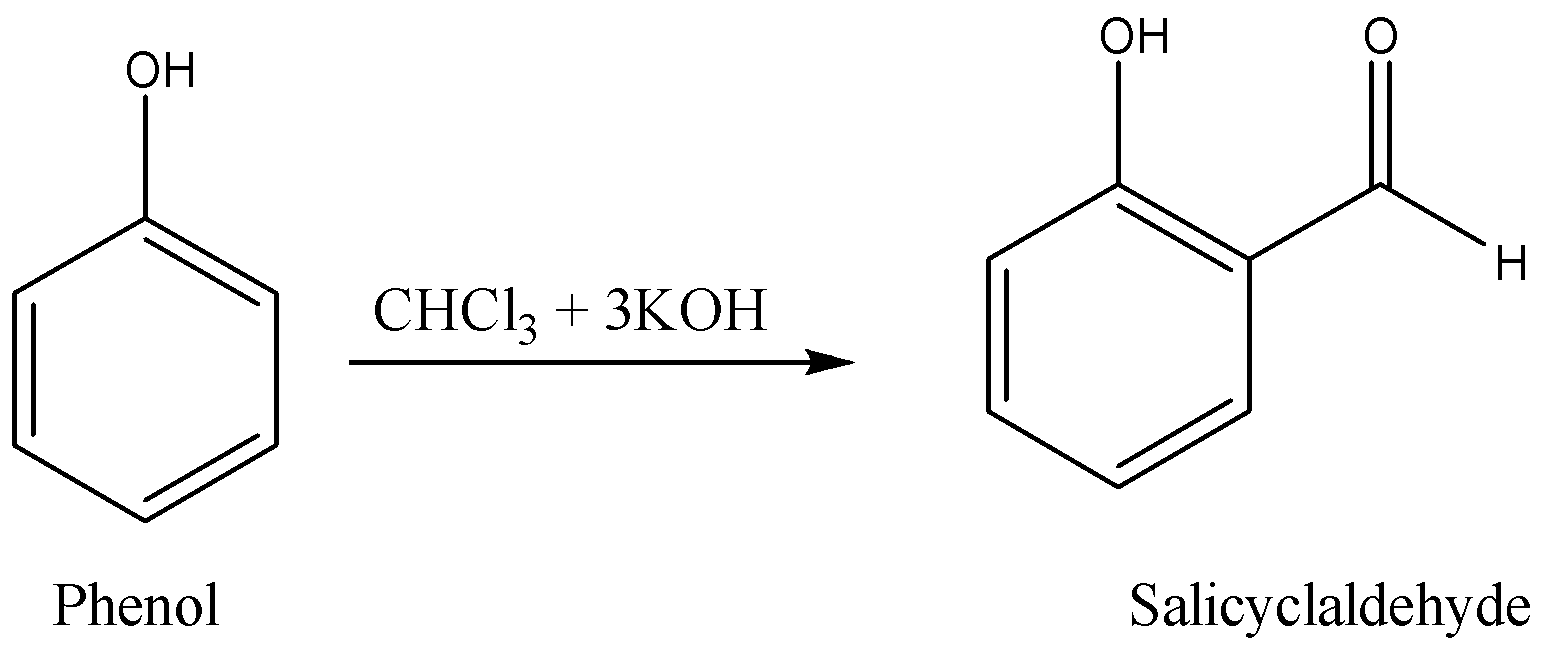
Reimer Tiemann reaction is a type of electrophilic substitution reaction. Reimer Tiemann reaction converts phenol to ortho-hydroxybenzaldehyde.
Complete step by step answer:
A simple reimer Tiemann reaction with reactants, reagents, and products can be written as given below.
The reaction mechanism is given below which involves the formation of dichlorocarbene which is represented as \[:CC{l_2}\].
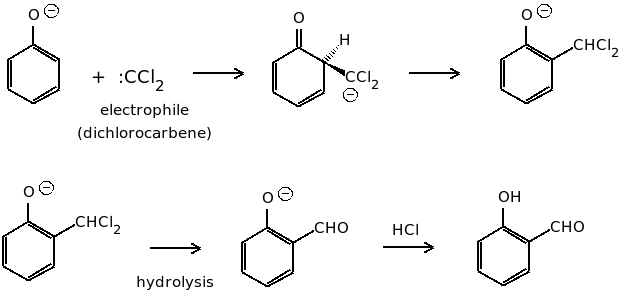
From the above reaction mechanism, we can conclude that the Reimer Tiemann reaction is an electrophilic substitution reaction in which dichlorocarbene acts as an electrophile and is substituted at the ortho position.
So, the correct answer is Option B.
Note: Electrophilic substitution reaction is the type of chemical reaction in which an electrophile displaces any functional group present at any position to form a product.
There is one more type of substitution reaction called nucleophilic substitution reaction in which the leaving group is replaced by the electron-rich compound.
The condition of the Reimer Tiemann reaction is that it requires \[1\] a mole of \[CHC{l_3}\] chloroform and \[3\]moles of potassium hydroxide \[KOH\].
Some of the examples of electrophiles are \[NO_2^ +,S{O_3}\].
Reimer Tiemann reaction is a type of electrophilic substitution reaction. Reimer Tiemann reaction converts phenol to ortho-hydroxybenzaldehyde.
Complete step by step answer:
A simple reimer Tiemann reaction with reactants, reagents, and products can be written as given below.

The reaction mechanism is given below which involves the formation of dichlorocarbene which is represented as \[:CC{l_2}\].

From the above reaction mechanism, we can conclude that the Reimer Tiemann reaction is an electrophilic substitution reaction in which dichlorocarbene acts as an electrophile and is substituted at the ortho position.
So, the correct answer is Option B.
Note: Electrophilic substitution reaction is the type of chemical reaction in which an electrophile displaces any functional group present at any position to form a product.
There is one more type of substitution reaction called nucleophilic substitution reaction in which the leaving group is replaced by the electron-rich compound.
The condition of the Reimer Tiemann reaction is that it requires \[1\] a mole of \[CHC{l_3}\] chloroform and \[3\]moles of potassium hydroxide \[KOH\].
Some of the examples of electrophiles are \[NO_2^ +,S{O_3}\].
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




