
In the reaction of p-chlorotoluene with $KN{H_2}$ in liquid $N{H_3}$, the major product is.
A) O-toluidine.
B) m-toluidine.
C) p-toluidine.
D) P-chloroaniline.
Answer
587.1k+ views
Hint: We know that the reaction of p-chlorotoluene with $KN{H_2}$ in liquid $N{H_3}$,proceeds via benzyne mechanism. The primary stage in the mechanism is a base promoted Dehydrohalogenation of chlorobenzene and the intermediate formed in the step will contain a triple bond in an aromatic ring called benzyne.
Complete step by step answer:
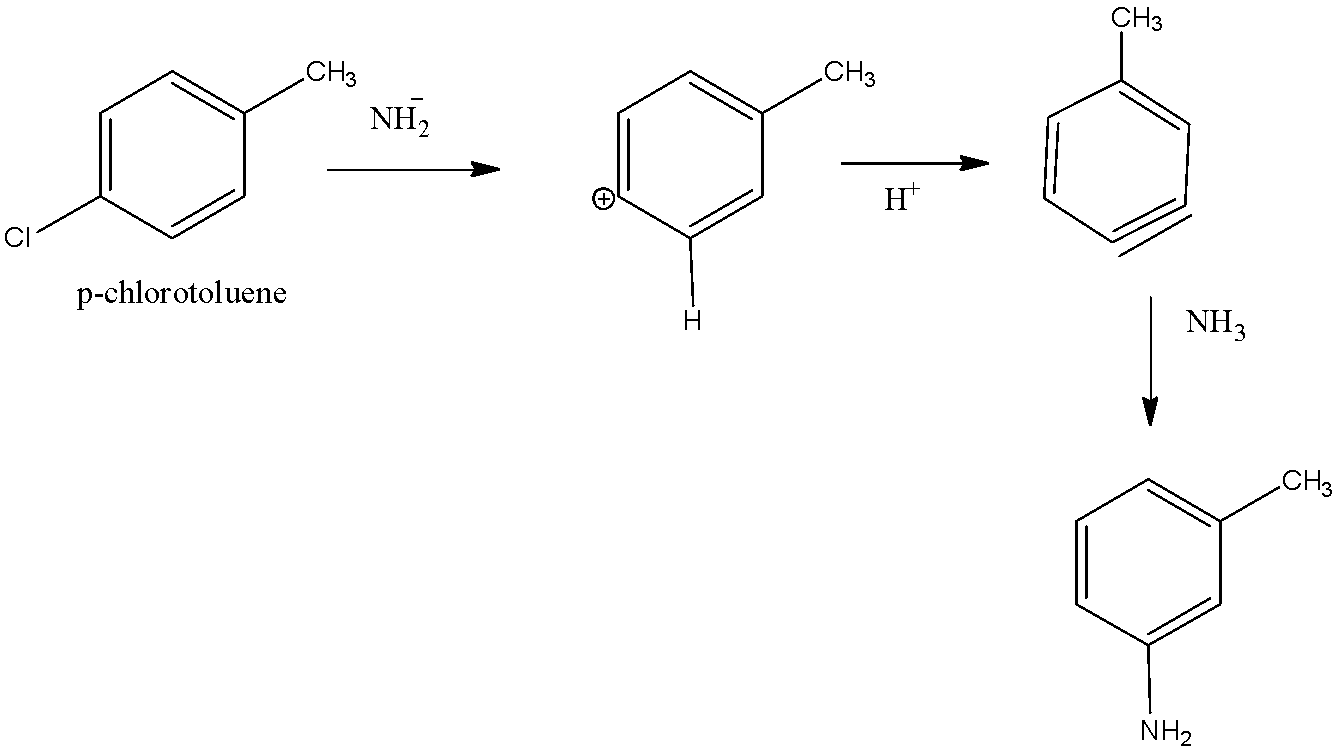
We know that para-Chlorotoluene initially forms benzyne with methyl as substituent. When -\[N{H_2}\] attacks at carbon which is bearing triple bond it gives a mixture of Meta and para-toluidine. The major product formed is m-toluidine because of the formation of carbanion at meta position by the attack of nucleophile and it is more stable compared to para position. Hence option B is correct.
The mechanism at which the reaction takes place,
First step: Removal of proton by \[KN{H_2}\] from the Meta position of p-chlorotoluene which provides benzyne as an intermediate.
Second step: Since benzyne is susceptible to nucleophilic attack and hence the nucleophile present within the solution is \[N{H_2}\]which may attack on the each side of the Benzene bond
Meta product is major as just in case of attack on 1 the negative charge formed is going to be farthest from the methyl group which facilitates less $ + 1$ and hence more stable negative ions.
We can write chemical equation for this mechanism as,
So, the correct answer is Option B.
Note:
Two mechanisms were proposed for the nucleophilic aromatic substitution, one among the mechanisms involves a benzyne because the intermediate formed is a benzyne and so, it is named as benzyne mechanism. Arynes or benzyne are extremely reactive groups resulting from an aromatic ring by elimination of two substituents.
Complete step by step answer:
We know that para-Chlorotoluene initially forms benzyne with methyl as substituent. When -\[N{H_2}\] attacks at carbon which is bearing triple bond it gives a mixture of Meta and para-toluidine. The major product formed is m-toluidine because of the formation of carbanion at meta position by the attack of nucleophile and it is more stable compared to para position. Hence option B is correct.
The mechanism at which the reaction takes place,
First step: Removal of proton by \[KN{H_2}\] from the Meta position of p-chlorotoluene which provides benzyne as an intermediate.
Second step: Since benzyne is susceptible to nucleophilic attack and hence the nucleophile present within the solution is \[N{H_2}\]which may attack on the each side of the Benzene bond
Meta product is major as just in case of attack on 1 the negative charge formed is going to be farthest from the methyl group which facilitates less $ + 1$ and hence more stable negative ions.
We can write chemical equation for this mechanism as,

So, the correct answer is Option B.
Note:
Two mechanisms were proposed for the nucleophilic aromatic substitution, one among the mechanisms involves a benzyne because the intermediate formed is a benzyne and so, it is named as benzyne mechanism. Arynes or benzyne are extremely reactive groups resulting from an aromatic ring by elimination of two substituents.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




