
In the reaction :
${\text{M}}{{\text{g}}_{\text{2}}}{{\text{C}}_{\text{3}}}$ reacts with water to form propyne. ${\text{C}}_3^{4 - }$ has:
A) Two sigma and two pi bonds
B) Three sigma and one pi bonds.
C) Two sigma and one pi bonds.
D) Two sigma and three pi bonds.
Answer
564.6k+ views
Hint: We know that ${\text{M}}{{\text{g}}_{\text{2}}}{{\text{C}}_{\text{3}}}$ is magnesium carbide. Metal carbides in reaction with water are generally used to produce alkynes. Magnesium carbide reacts with water to form propyne and magnesium hydroxide.
Complete step by step answer:
We know that ${\text{M}}{{\text{g}}_{\text{2}}}{{\text{C}}_{\text{3}}}$ is magnesium carbide. Metal carbides in reaction with water are generally used to produce alkynes. Magnesium carbide reacts with water to form propyne and magnesium hydroxide.
-The reaction of magnesium carbide with water is as follows:
${\text{M}}{{\text{g}}_{\text{2}}}{{\text{C}}_{\text{3}}} + 4{{\text{H}}_2}{\text{O}} \to {\text{C}}{{\text{H}}_3} - {\text{C}} \equiv {\text{CH}} + 2{\text{Mg}}{\left( {{\text{OH}}} \right)_2}$
-The reaction is used as a preparation reaction of propyne. Propyne is produced because magnesium carbide contains three carbon atoms.
-Propyne is ${\text{C}}{{\text{H}}_3} - {\text{C}} \equiv {\text{CH}}$. When four hydrogen atoms are removed from the propyne, ${\text{C}}_3^{4 - }$ is obtained. The reaction is as follows:
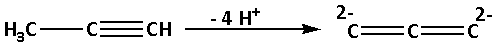
The structure of ${\text{C}}_3^{4 - }$ is as follows:
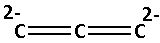
Now, we have to determine the bonds in ${\text{C}}_3^{4 - }$.
-The single bonds in a compound are known as the sigma $\left( {\text{\sigma }} \right)$ bonds. If there are double bonds in a compound then one bond is sigma $\left( {\text{\sigma }} \right)$ bond and one is the pi $\left( {\text{\pi }} \right)$ bond. If there are triple bonds in a compound then one bond is the sigma $\left( {\text{\sigma }} \right)$ bond and two are the pi $\left( {\text{\pi }} \right)$ bonds.
-The ${\text{C}}_3^{4 - }$ there are two double bonds. If there are double bonds in a compound then one bond is sigma $\left( {\text{\sigma }} \right)$ bond and one is the pi $\left( {\text{\pi }} \right)$ bond. Thus, there are two sigma bonds and two pi bonds in ${\text{C}}_3^{4 - }$.
Thus, ${\text{C}}_3^{4 - }$ has two sigma and two pi bonds.
Thus, the correct option is (A).
Note: The sigma bond overlapping is very large and thus, the sigma bond is stronger. The overlapping of pi bonds is less and thus, pi bond is weaker. The sigma bond has one-electron cloud and the pi bond has a two-electron cloud.
Complete step by step answer:
We know that ${\text{M}}{{\text{g}}_{\text{2}}}{{\text{C}}_{\text{3}}}$ is magnesium carbide. Metal carbides in reaction with water are generally used to produce alkynes. Magnesium carbide reacts with water to form propyne and magnesium hydroxide.
-The reaction of magnesium carbide with water is as follows:
${\text{M}}{{\text{g}}_{\text{2}}}{{\text{C}}_{\text{3}}} + 4{{\text{H}}_2}{\text{O}} \to {\text{C}}{{\text{H}}_3} - {\text{C}} \equiv {\text{CH}} + 2{\text{Mg}}{\left( {{\text{OH}}} \right)_2}$
-The reaction is used as a preparation reaction of propyne. Propyne is produced because magnesium carbide contains three carbon atoms.
-Propyne is ${\text{C}}{{\text{H}}_3} - {\text{C}} \equiv {\text{CH}}$. When four hydrogen atoms are removed from the propyne, ${\text{C}}_3^{4 - }$ is obtained. The reaction is as follows:
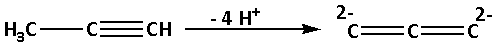
The structure of ${\text{C}}_3^{4 - }$ is as follows:
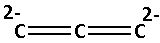
Now, we have to determine the bonds in ${\text{C}}_3^{4 - }$.
-The single bonds in a compound are known as the sigma $\left( {\text{\sigma }} \right)$ bonds. If there are double bonds in a compound then one bond is sigma $\left( {\text{\sigma }} \right)$ bond and one is the pi $\left( {\text{\pi }} \right)$ bond. If there are triple bonds in a compound then one bond is the sigma $\left( {\text{\sigma }} \right)$ bond and two are the pi $\left( {\text{\pi }} \right)$ bonds.
-The ${\text{C}}_3^{4 - }$ there are two double bonds. If there are double bonds in a compound then one bond is sigma $\left( {\text{\sigma }} \right)$ bond and one is the pi $\left( {\text{\pi }} \right)$ bond. Thus, there are two sigma bonds and two pi bonds in ${\text{C}}_3^{4 - }$.
Thus, ${\text{C}}_3^{4 - }$ has two sigma and two pi bonds.
Thus, the correct option is (A).
Note: The sigma bond overlapping is very large and thus, the sigma bond is stronger. The overlapping of pi bonds is less and thus, pi bond is weaker. The sigma bond has one-electron cloud and the pi bond has a two-electron cloud.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




