
In the reaction
$H - C \equiv CH\xrightarrow[{\left( 2 \right)C{H_3}C{H_2}Br}]{{\left( 1 \right)NaN{H_2}/liq.N{H_3}}}X\xrightarrow[{\left( 2 \right)C{H_3}C{H_2}Br}]{{\left( 1 \right)NaN{H_2}/liq.N{H_3}}}Y$. X and Y are.
A) $X = 1 - Butyne$ , $Y = 3 - Hexyne$
B) $X = 2 - Butyne$ , $Y = 3 - Hexyne$
C) $X = 2 - Butyne$ , $Y = 2 - Hexyne$
D) $X = 1 - Butyne$ , $Y = 2 - Hexyne$
Answer
563.1k+ views
Hint: We have to remember that the terminal acetylenic compounds having acidic hydrogen react with sodium amide to form sodium acetylenes which may be converted to acids and higher acetylenes. We also remember that the sodamide is the most commonly used base for the formation of triple bonds from suitable alkyl halides.
Complete step by step answer:
We must remember that the sodium metal itself, Na, may be a one-electron reducer – it's oxidized to \[N{a^ + }\] within the process. When it reduces neutral hydrocarbons, charged species are formed, which devour a proton from the ammonia solvent. Overall, \[Na/N{H_3}\] may be a way of reducing some organic compounds. Sodium amide is a useful reagent and it is prepared by reacting metallic sodium with liquid ammonia in the presence of nitrate. It finds use in various reactions in which reacting species is the amide anion.
Sodium amide may be a strong base which reacts with terminal alkynes and removes the terminal acid hydrogen leaving alkynes as a carbanion, which in reaction with haloalkane produces longer alkynes by nucleophilic addition. Thus, $X = 1 - Butyne$ , $Y = 3 - Hexyne$.
We can write the given reaction as below,
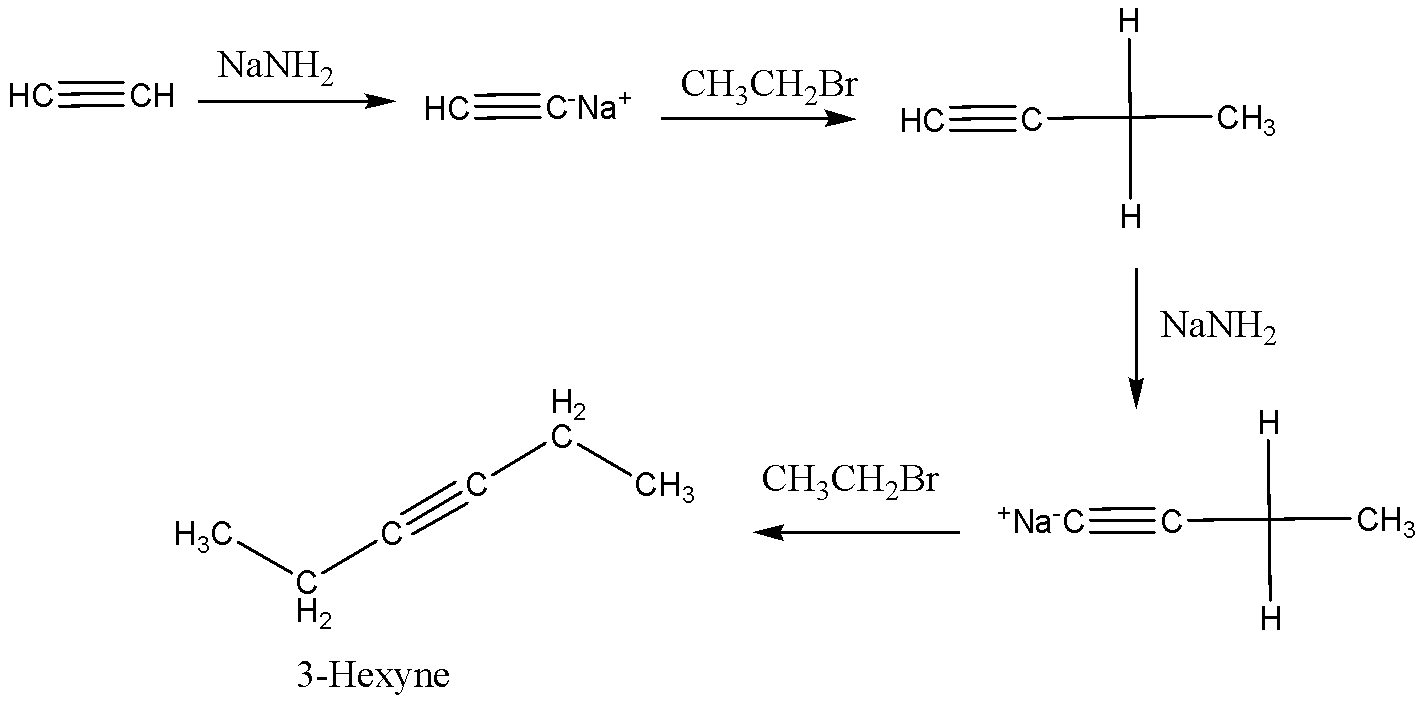
Therefore, the option A is correct.
Note: Now we discuss about the dehydrohalogenation of alkyl halides as,
We need to know that the sodium amide induces the loss of two bromide ions from a vicinal dibromoalkane to give a carbon-carbon triple bond during a preparation of phenylacetylene. Generally two equivalents of sodium amide yield the specified alkynes. Three equivalents are necessary within the preparation of a terminal alkyne because the terminal \[CH\] of the resulting alkynes protonated the same amount of base.
Complete step by step answer:
We must remember that the sodium metal itself, Na, may be a one-electron reducer – it's oxidized to \[N{a^ + }\] within the process. When it reduces neutral hydrocarbons, charged species are formed, which devour a proton from the ammonia solvent. Overall, \[Na/N{H_3}\] may be a way of reducing some organic compounds. Sodium amide is a useful reagent and it is prepared by reacting metallic sodium with liquid ammonia in the presence of nitrate. It finds use in various reactions in which reacting species is the amide anion.
Sodium amide may be a strong base which reacts with terminal alkynes and removes the terminal acid hydrogen leaving alkynes as a carbanion, which in reaction with haloalkane produces longer alkynes by nucleophilic addition. Thus, $X = 1 - Butyne$ , $Y = 3 - Hexyne$.
We can write the given reaction as below,

Therefore, the option A is correct.
Note: Now we discuss about the dehydrohalogenation of alkyl halides as,
We need to know that the sodium amide induces the loss of two bromide ions from a vicinal dibromoalkane to give a carbon-carbon triple bond during a preparation of phenylacetylene. Generally two equivalents of sodium amide yield the specified alkynes. Three equivalents are necessary within the preparation of a terminal alkyne because the terminal \[CH\] of the resulting alkynes protonated the same amount of base.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




