
In the process of $ BC{l_3} + P{H_3} \to \,BC{l_3}\,:P{H_3} $ . The Lewis acid is
A. $ P{H_3} $
B. $ BC{l_3} $
C. Both
D. None
Answer
527.4k+ views
Hint :G. N. Lewis proposed a different way of looking at the reaction between $ {H^ + } $ and $ O{H^ - } $ ions in 1923. The $ O{H^ - } $ ion is the active species in this reaction, accepting an $ {H^ + } $ ion to form a covalent bond, according to the Bronsted model. The active species in the Lewis model is the H+ ion, which accepts a pair of electrons from the $ O{H^ - } $ ion to form a covalent bond.
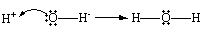
Complete Step By Step Answer:
Bases donate pairs of electrons to acids, and acids accept pairs of electrons, according to the Lewis principle of acid-base reactions. Any material that can accommodate a pair of nonbonding electrons, such as the H+ ion, is referred to as a Lewis acid. A Lewis acid, in other words, is an electron-pair acceptor. A Lewis base is any material that can donate a pair of nonbonding electrons, such as the OH- ion. A Lewis base, as a result, is an electron-pair donor.
The Lewis theory has the advantage of complementing the oxidation-reduction model. The movement of electrons from one atom to another in oxidation-reduction reactions results in a net change in the oxidation number of one or more atoms.
According to the Lewis principle, acids and bases share a pair of electrons without changing the oxidation numbers of any atoms. Many chemical reactions can be classified into one of these two groups. Either electrons are exchanged between atoms or the atoms come together to share a pair of electrons.
Now, coming to the question in the above given reaction $ BC{l_3} $ is the Lewis acid because It accepts the electron pair from the $ P{H_3} $ .Since $ BC{l_3} $ is an electron-deficient molecule (B has just 6 electrons), it behaves as a Lewis acid, accepting a pair of electrons to complete its octet. This is based on the Lewis principle, which states that any positively charged or electron deficient species acts as a Lewis acid.
So, the correct answer is option: (B) $ BC{l_3} $ .
Note :
The Lewis theory's main benefit is that it increases the number of acids and hence the number of acid-base reactions. An acid, according to Lewis' theory, is any ion or molecule that can accommodate two nonbonding valence electrons. We concluded in the previous section that $Al^{3+}$ ions form bonds with six water molecules to form a complex ion.
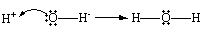
Complete Step By Step Answer:
Bases donate pairs of electrons to acids, and acids accept pairs of electrons, according to the Lewis principle of acid-base reactions. Any material that can accommodate a pair of nonbonding electrons, such as the H+ ion, is referred to as a Lewis acid. A Lewis acid, in other words, is an electron-pair acceptor. A Lewis base is any material that can donate a pair of nonbonding electrons, such as the OH- ion. A Lewis base, as a result, is an electron-pair donor.
The Lewis theory has the advantage of complementing the oxidation-reduction model. The movement of electrons from one atom to another in oxidation-reduction reactions results in a net change in the oxidation number of one or more atoms.
According to the Lewis principle, acids and bases share a pair of electrons without changing the oxidation numbers of any atoms. Many chemical reactions can be classified into one of these two groups. Either electrons are exchanged between atoms or the atoms come together to share a pair of electrons.
Now, coming to the question in the above given reaction $ BC{l_3} $ is the Lewis acid because It accepts the electron pair from the $ P{H_3} $ .Since $ BC{l_3} $ is an electron-deficient molecule (B has just 6 electrons), it behaves as a Lewis acid, accepting a pair of electrons to complete its octet. This is based on the Lewis principle, which states that any positively charged or electron deficient species acts as a Lewis acid.
So, the correct answer is option: (B) $ BC{l_3} $ .
Note :
The Lewis theory's main benefit is that it increases the number of acids and hence the number of acid-base reactions. An acid, according to Lewis' theory, is any ion or molecule that can accommodate two nonbonding valence electrons. We concluded in the previous section that $Al^{3+}$ ions form bonds with six water molecules to form a complex ion.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




