
In the given reaction the product P is:
A)
B)
C)
D)





Answer
578.1k+ views
Hint: The cyclohexane reacts with hydrogen fluoride to form a cation. Benzene gives a substitution reaction with this cation. The product has two joined rings.
Complete step by step answer:
To attach substituents on aromatic rings the Friedel craft alkylation reactions are used. Friedel craft alkylation reactions are the reaction of aromatic rings with alkyl groups in presence of Lewis acid.
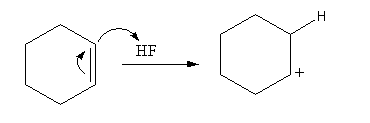
Firstly, the cyclohexene reacts with acid hydrogen fluoride, so cyclohexene abstract protons from the hydrogen fluoride form a cation of cyclohexane.
The reaction is shown as follows:
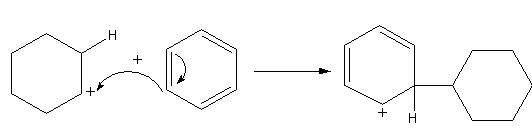
The benzene attacks on the cyclohexyl cation to form the cation again. The attack of benzene attacks on the cyclohexane joins the two rings or we can say alkylate the benzene ring.
The reaction is shown as follows:
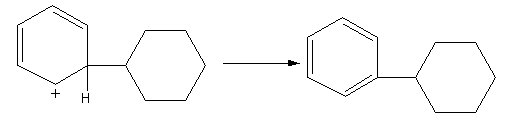
The positive charge is stabilized by the delocalization in the benzene ring. The abstraction of hydrogen from the benzene ring gives the final product.
The product is shown as follows:
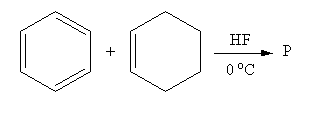
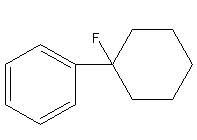
So, the product P of the reaction of benzene with cyclohexene in presence of Lewis acid hydrogen fluoride is 1-cyclohexylbenzene.
Therefore, option (A) is correct.
Note: The given reaction of cyclohexane and benzene in presence of hydrogen fluoride is an example of Friedel craft alkylation. Which is an electrophilic aromatic substitution type reaction. The alkyl groups are electron donating groups, so increases the nucleophilicity of the species. So, the product of Friedel craft alkylation is more nucleophilic than the reactant.
Complete step by step answer:
To attach substituents on aromatic rings the Friedel craft alkylation reactions are used. Friedel craft alkylation reactions are the reaction of aromatic rings with alkyl groups in presence of Lewis acid.
Firstly, the cyclohexene reacts with acid hydrogen fluoride, so cyclohexene abstract protons from the hydrogen fluoride form a cation of cyclohexane.
The reaction is shown as follows:

The benzene attacks on the cyclohexyl cation to form the cation again. The attack of benzene attacks on the cyclohexane joins the two rings or we can say alkylate the benzene ring.
The reaction is shown as follows:

The positive charge is stabilized by the delocalization in the benzene ring. The abstraction of hydrogen from the benzene ring gives the final product.
The product is shown as follows:

So, the product P of the reaction of benzene with cyclohexene in presence of Lewis acid hydrogen fluoride is 1-cyclohexylbenzene.
Therefore, option (A) is correct.
Note: The given reaction of cyclohexane and benzene in presence of hydrogen fluoride is an example of Friedel craft alkylation. Which is an electrophilic aromatic substitution type reaction. The alkyl groups are electron donating groups, so increases the nucleophilicity of the species. So, the product of Friedel craft alkylation is more nucleophilic than the reactant.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




