
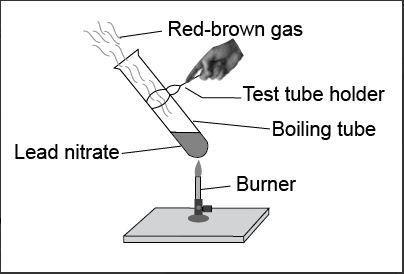
In the given figure, the red brown gas evolved is____.

Answer
580.8k+ views
Hint: Here we can see that lead nitrate is being burned and a gas is produced due to combustion which eventually can be understood to have the same root element as lead nitrate i.e. nitrogen. To make this confirm we should describe the whole process.
Complete answer:
Let us see the process in detail before we move towards the exact gas which would be in red brown colour when lead nitrate is burned.
Basically, lead nitrate (plumbous nitrate) is a white crystalline solid which is soluble in water but is non-combustible at normal temperatures. But when we heat the lead nitrate at above 470${}^\circ C$ it would not melt but decompose into products due to which this compound is used in fireworks.
Heating of lead nitrate produces lead monoxide, nitrogen dioxide gas and oxygen gas as products. The reaction is given as,
$2Pb{{\left( N{{O}_{3}} \right)}_{2}}\to 2PbO+4N{{O}_{2}}+{{O}_{2}}$
It is a decomposition reaction in which a compound breaks down into two or more simpler compounds i.e. lead nitrate decomposes to give lead monoxide, nitrogen dioxide and oxygen as the products.
Here, lead oxide is the residue which is yellowish solid in colour when hot and white in colour when cold. The oxygen gas is a colourless gas liberated along with lead oxide and nitrogen gas. The nitrogen gas is the reddish brown gas liberated which is toxic.
Thus, the reddish brown gas is nitrogen dioxide gas.
Note: Do note that the compound here i.e. lead nitrate is carcinogenic for humans causing most probably kidney disorders. Thus, the reaction carried out must be limited and proper precautions must be taken while performing.
Complete answer:
Let us see the process in detail before we move towards the exact gas which would be in red brown colour when lead nitrate is burned.
Basically, lead nitrate (plumbous nitrate) is a white crystalline solid which is soluble in water but is non-combustible at normal temperatures. But when we heat the lead nitrate at above 470${}^\circ C$ it would not melt but decompose into products due to which this compound is used in fireworks.
Heating of lead nitrate produces lead monoxide, nitrogen dioxide gas and oxygen gas as products. The reaction is given as,
$2Pb{{\left( N{{O}_{3}} \right)}_{2}}\to 2PbO+4N{{O}_{2}}+{{O}_{2}}$
It is a decomposition reaction in which a compound breaks down into two or more simpler compounds i.e. lead nitrate decomposes to give lead monoxide, nitrogen dioxide and oxygen as the products.
Here, lead oxide is the residue which is yellowish solid in colour when hot and white in colour when cold. The oxygen gas is a colourless gas liberated along with lead oxide and nitrogen gas. The nitrogen gas is the reddish brown gas liberated which is toxic.
Thus, the reddish brown gas is nitrogen dioxide gas.
Note: Do note that the compound here i.e. lead nitrate is carcinogenic for humans causing most probably kidney disorders. Thus, the reaction carried out must be limited and proper precautions must be taken while performing.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




