
In the given Dewar structure of benzene, which of the following statement(s), is correct?
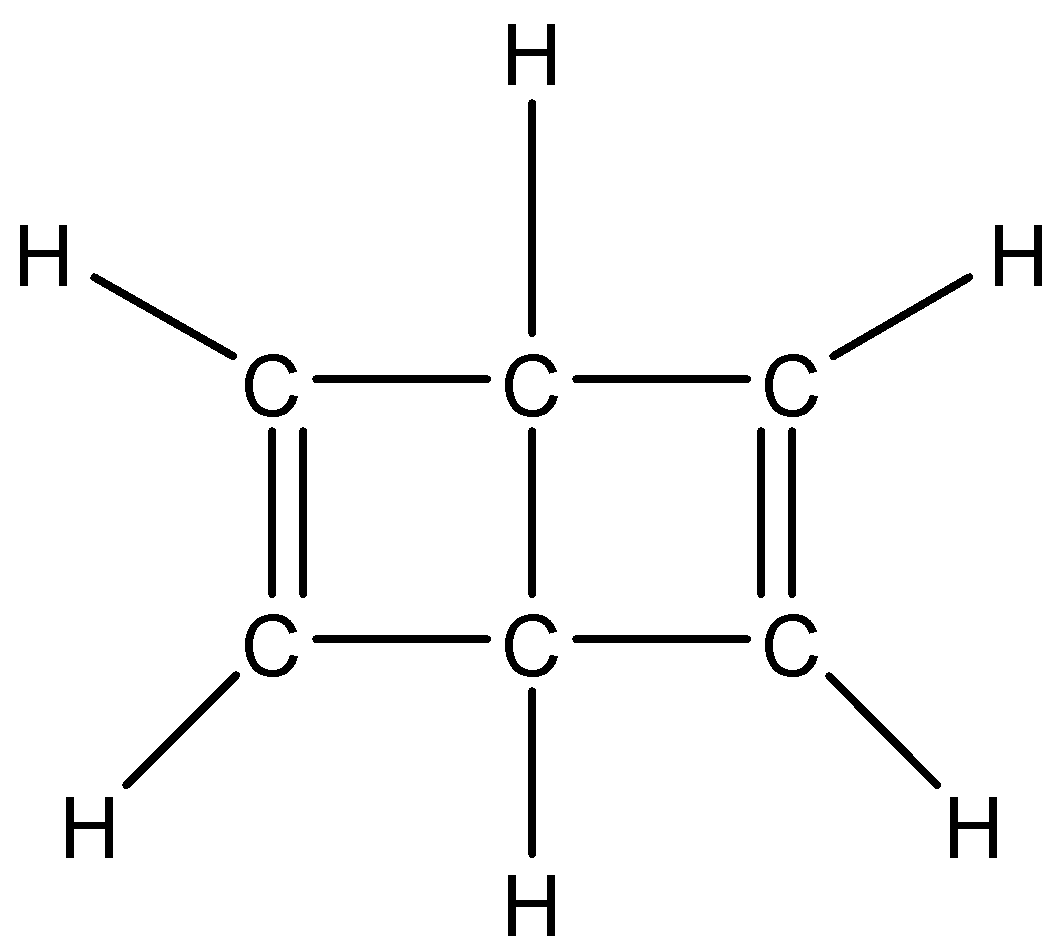
(A) All the carbons are in $s{p^2}$ hybrid state
(B) All the carbons are in $s{p^3}$ hybrid state
(C) Four carbons are in $s{p^2}$ and two in $s{p^3}$ hybrid state
(D) Four carbons are in $s{p^3}$ and two in $s{p^2}$ hybrid state

Answer
564.6k+ views
Hint:In the given options we are informed about the hybridization state of carbons in the molecule, so we have to configure the correct hybridization state of every Carbon atom. Hybridization is basically the mixing of atomic orbitals to form new types of orbitals.
Complete answer:
Hybridization is the phenomenon in which atomic orbitals that have similar energy are combined together to make new atomic orbitals with different structures and properties. The new orbitals that are formed after the mixing are called ‘Hybrid Orbitals’. Based on mixing hybridization is divided into $6$ types, these are $sp,\,s{p^2},s{p^3},s{p^3}d,s{p^3}{d^2},{d^2}s{p^3}$.
Carbon atom shows only three types of Hybridization i.e. $sp,\,s{p^2},s{p^3}\,$. The $sp$ hybridization of carbon is shown when it is bonded with two atoms with $2$double bonds or it is bonded with $1$ single and $1$ triple bonds. The type of arrangement in molecules with $sp$ hybridization is linear with a bond angle of ${180^0}$.
Carbon shows $s{p^2}$hybridisation when there is a mixing of $1s$ orbital and two $2p$ orbitals. In this carbon forms two single bonds and one double bond with a total three atoms. The orbitals have a triangular geometry with a bond angle of ${120^0}$.
Carbon shows $s{p^3}$ hybridisation when there is a mixing of $1s$ orbital and three $3p$ orbitals. In this carbon forms four single bonds with four atoms. The orbitals have a tetrahedral geometry with a bond angle of ${109.5^0}$.
Now, in the given dewar structure of benzene, we can see that four carbon atoms are bonded with one double bond and two single bonds, and two carbon atoms are bonded with four single bonds with hydrogen and carbon.
Hence four carbons will exhibit $s{p^2}$ hybridisation and two will exhibit $s{p^3}$ hybridisation.
Therefore, option (C) is correct.
Note:
Dewar Benzene is the bicyclic isomer of benzene. It was given by James Dewar. In this structure, the carbon atoms form a tetrahedral geometry rather than making a planar structure. This is due to the fact that the carbon atom where the ring is joined is bonded to four atoms instead of three.
Complete answer:
Hybridization is the phenomenon in which atomic orbitals that have similar energy are combined together to make new atomic orbitals with different structures and properties. The new orbitals that are formed after the mixing are called ‘Hybrid Orbitals’. Based on mixing hybridization is divided into $6$ types, these are $sp,\,s{p^2},s{p^3},s{p^3}d,s{p^3}{d^2},{d^2}s{p^3}$.
Carbon atom shows only three types of Hybridization i.e. $sp,\,s{p^2},s{p^3}\,$. The $sp$ hybridization of carbon is shown when it is bonded with two atoms with $2$double bonds or it is bonded with $1$ single and $1$ triple bonds. The type of arrangement in molecules with $sp$ hybridization is linear with a bond angle of ${180^0}$.
Carbon shows $s{p^2}$hybridisation when there is a mixing of $1s$ orbital and two $2p$ orbitals. In this carbon forms two single bonds and one double bond with a total three atoms. The orbitals have a triangular geometry with a bond angle of ${120^0}$.
Carbon shows $s{p^3}$ hybridisation when there is a mixing of $1s$ orbital and three $3p$ orbitals. In this carbon forms four single bonds with four atoms. The orbitals have a tetrahedral geometry with a bond angle of ${109.5^0}$.
Now, in the given dewar structure of benzene, we can see that four carbon atoms are bonded with one double bond and two single bonds, and two carbon atoms are bonded with four single bonds with hydrogen and carbon.
Hence four carbons will exhibit $s{p^2}$ hybridisation and two will exhibit $s{p^3}$ hybridisation.
Therefore, option (C) is correct.
Note:
Dewar Benzene is the bicyclic isomer of benzene. It was given by James Dewar. In this structure, the carbon atoms form a tetrahedral geometry rather than making a planar structure. This is due to the fact that the carbon atom where the ring is joined is bonded to four atoms instead of three.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




