
In the following thermal reaction, which side of the equilibrium is favored and why?
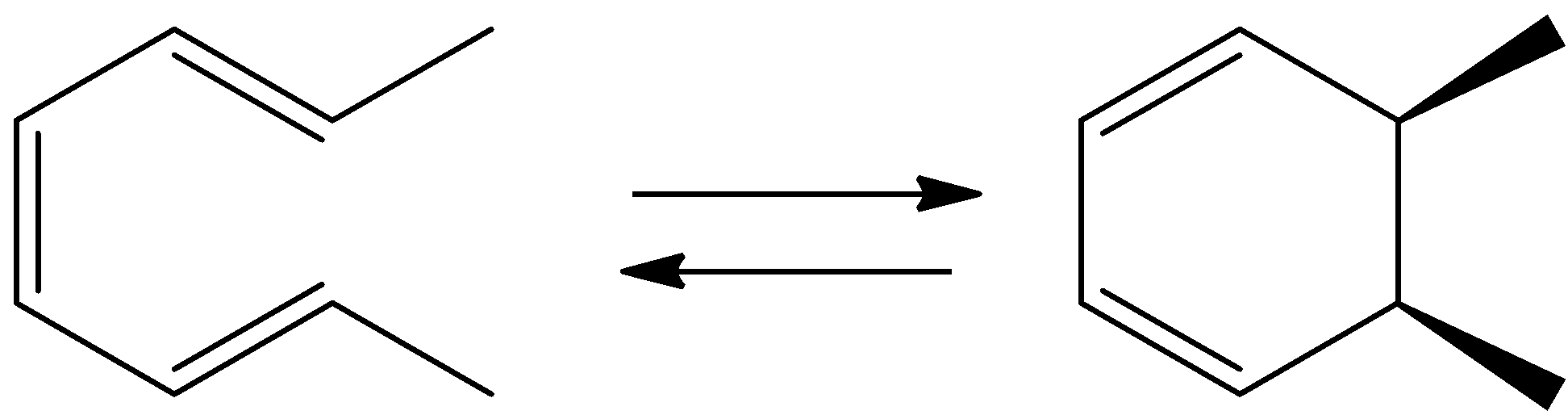
A.The product is favored because it is aromatic
B.The product is favored because there are more $C - C$ single bonds
C.The starting material is favored because ring strain is relieved
D.The starting material is favored due to orbital symmetry rules

Answer
590.1k+ views
Hint: We can predict the side which favors the equilibrium by knowing the amount of strain present in the compound.
We have to know that ring strain is a kind of instability which is seen when bonds in a molecule form abnormal angles. Strain is mostly seen in small rings such as cyclopropanes and cyclobutanes.
A combination of angle strain, conformational strain and trans annular strain leads to ring strain.
Complete step by step answer:
Let us know that some of the molecules that contain high amounts of ring strain include cyclopropanes, cyclopropenes, cyclobutanes, cyclobutanes, epoxides, aziridines, cyclopentanes, and norbornenes.
The ring structures of cyclopropanes/enes and cyclobutanes/enes show little conformational flexibility. Therefore, the substituents of ring atoms are found in an eclipsed conformation in cyclopropanes and between gauche and eclipsed in cyclobutanes, contributing to larger ring strain energy in the form of Van der Waals repulsion.
Ring strain could be higher in bicyclic systems.
Octa-2,4,6-triene undergoes reaction to form 5,6-dimethylcyclohexa-1,3-diene.
We can draw the chemical reaction as,
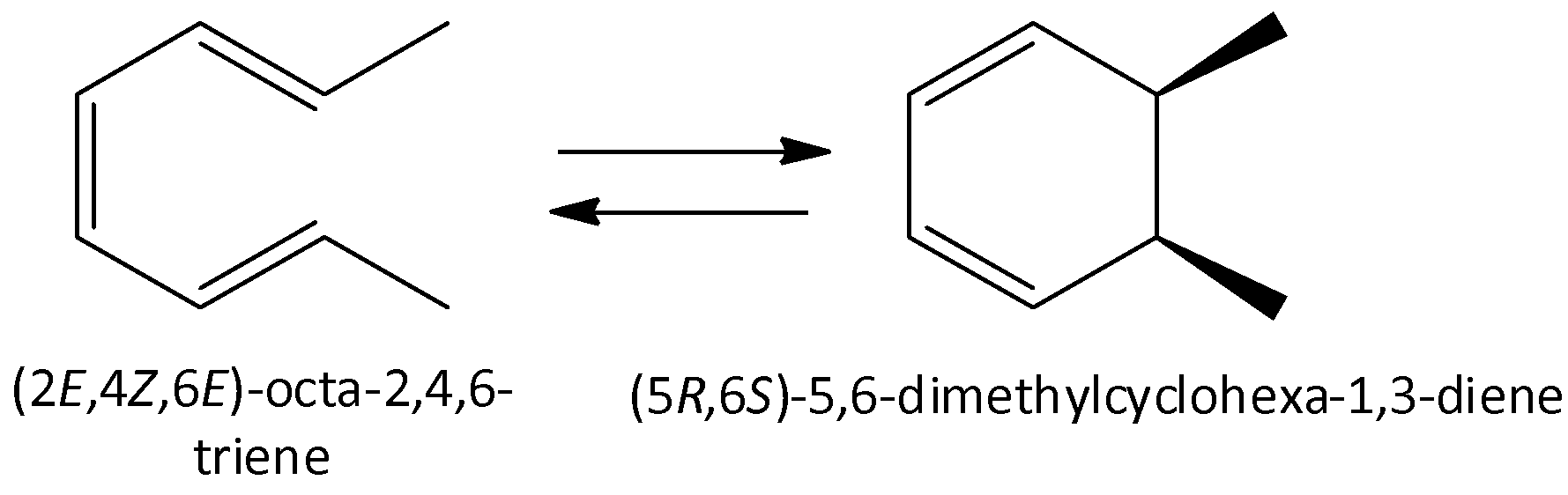
We know that open chain compounds contain less strain and are more stable when compared to the nonaromatic cyclic hexane. Non Aromatic cyclic hexane is less stable. Therefore, the starting material is more favorable due to lesser ring strain.
So, the correct answer is Option C .
Note:
The potential energy and unique bonding structure present in the bonds of molecules that contain ring strain could be used to progress reactions in organic synthesis. Ring opening metathesis polymerisation, photo-induced ring opening of cyclobutenes, and nucleophilic ring-opening of epoxides and aziridines are some of the examples of these kinds of reactions.
We have to know that ring strain is a kind of instability which is seen when bonds in a molecule form abnormal angles. Strain is mostly seen in small rings such as cyclopropanes and cyclobutanes.
A combination of angle strain, conformational strain and trans annular strain leads to ring strain.
Complete step by step answer:
Let us know that some of the molecules that contain high amounts of ring strain include cyclopropanes, cyclopropenes, cyclobutanes, cyclobutanes, epoxides, aziridines, cyclopentanes, and norbornenes.
The ring structures of cyclopropanes/enes and cyclobutanes/enes show little conformational flexibility. Therefore, the substituents of ring atoms are found in an eclipsed conformation in cyclopropanes and between gauche and eclipsed in cyclobutanes, contributing to larger ring strain energy in the form of Van der Waals repulsion.
Ring strain could be higher in bicyclic systems.
Octa-2,4,6-triene undergoes reaction to form 5,6-dimethylcyclohexa-1,3-diene.
We can draw the chemical reaction as,

We know that open chain compounds contain less strain and are more stable when compared to the nonaromatic cyclic hexane. Non Aromatic cyclic hexane is less stable. Therefore, the starting material is more favorable due to lesser ring strain.
So, the correct answer is Option C .
Note:
The potential energy and unique bonding structure present in the bonds of molecules that contain ring strain could be used to progress reactions in organic synthesis. Ring opening metathesis polymerisation, photo-induced ring opening of cyclobutenes, and nucleophilic ring-opening of epoxides and aziridines are some of the examples of these kinds of reactions.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




