
In the following reaction, the product S is:
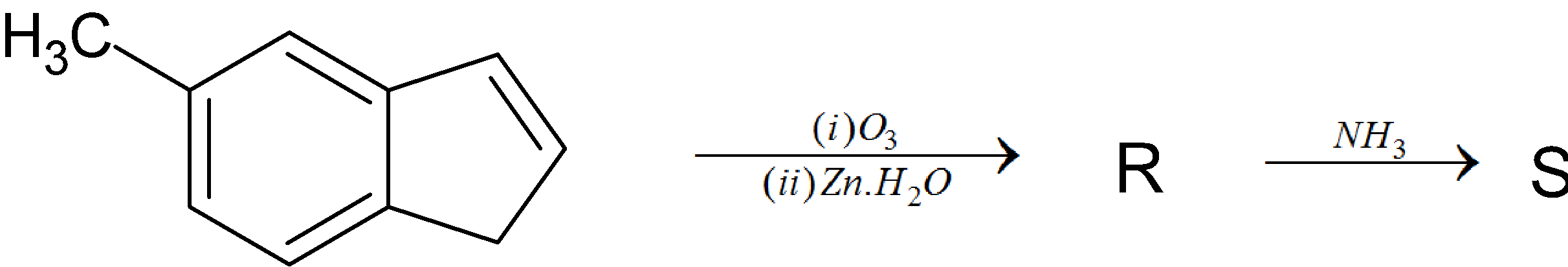
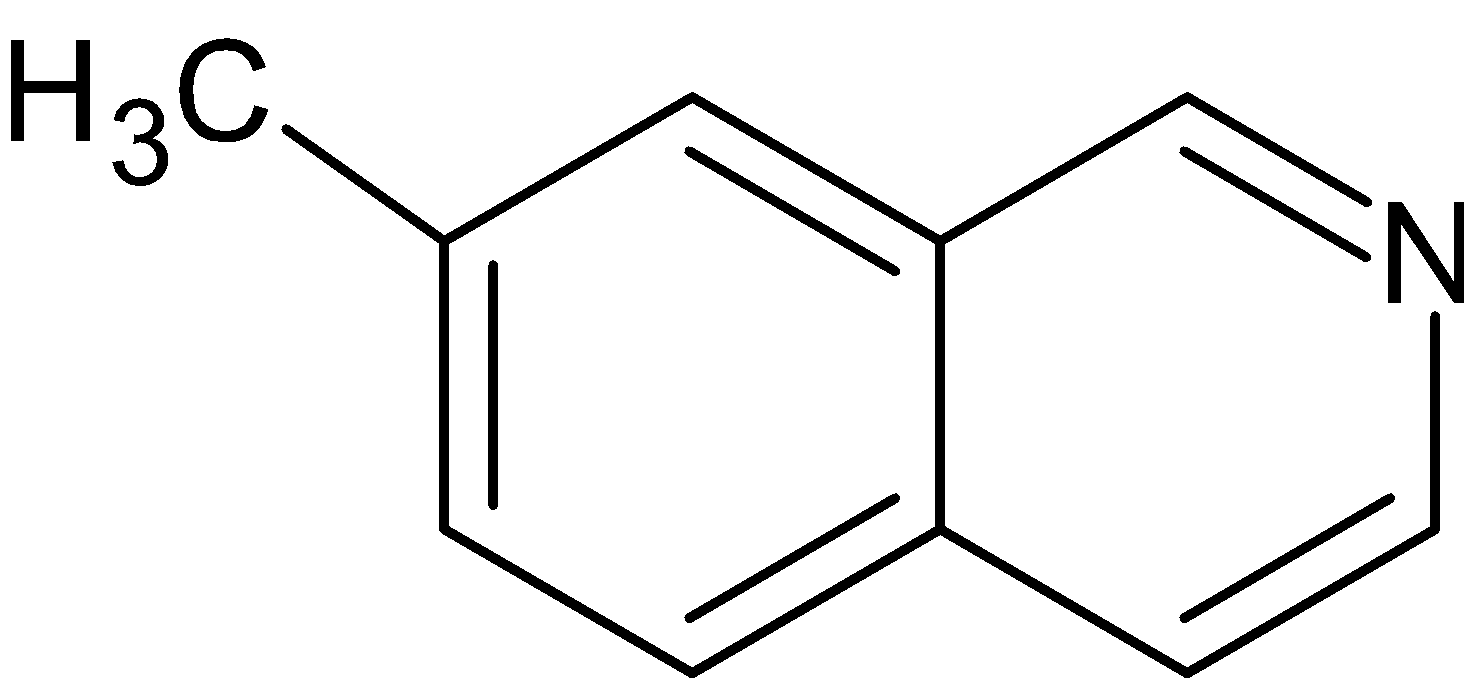
(A)
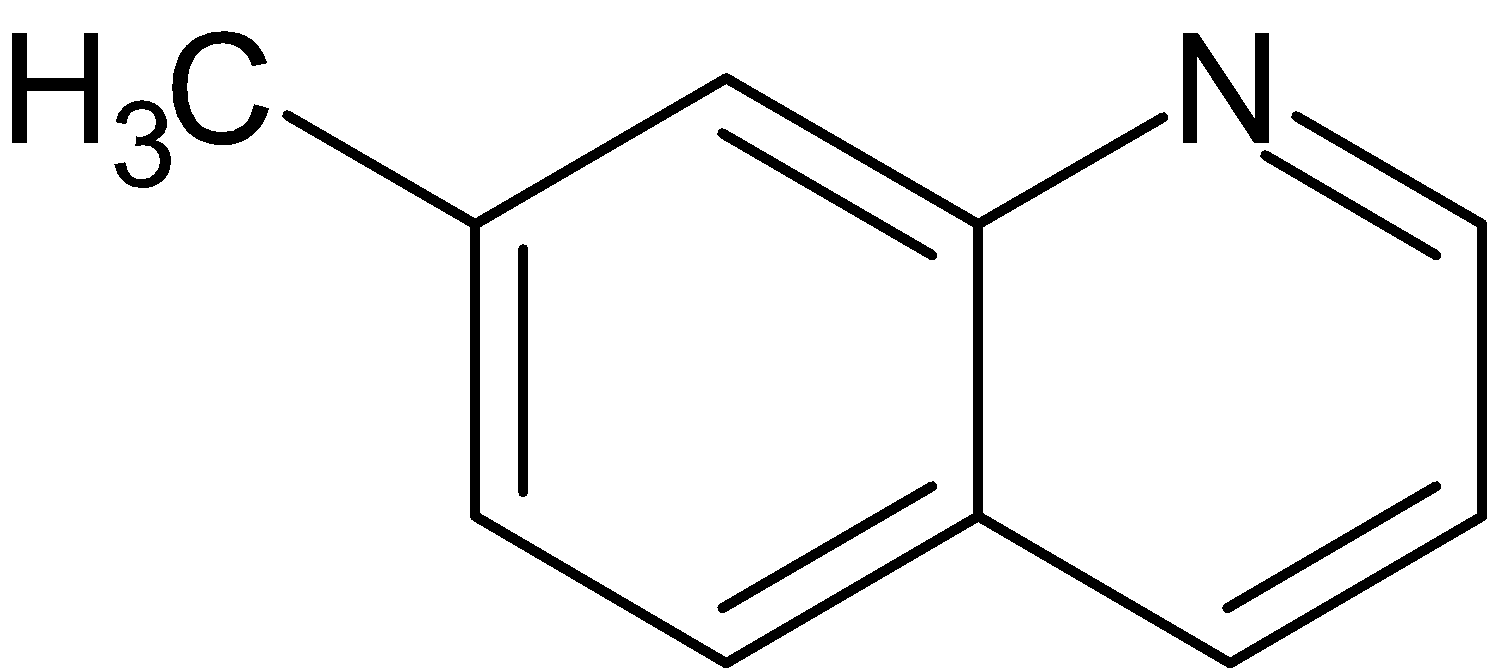
(B)
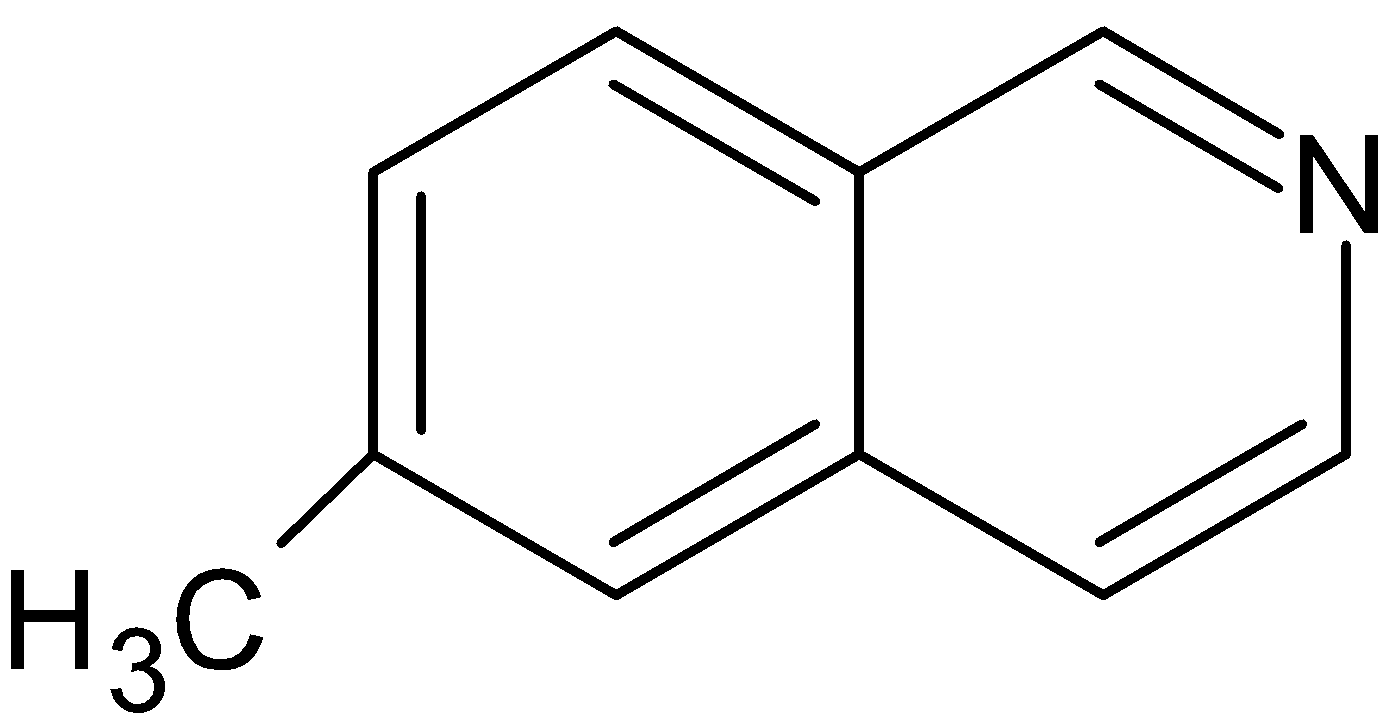
(C)
(D)
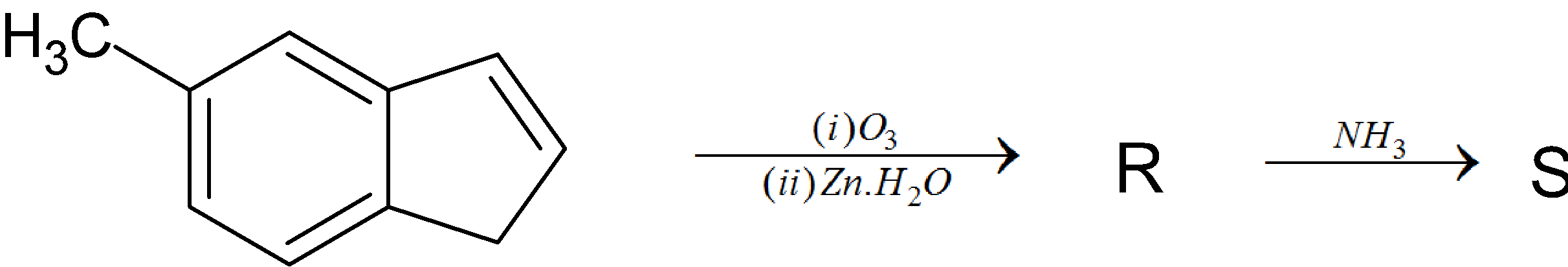




Answer
578.1k+ views
Hint: To solve this question we should know about ozonolysis, reductive workup. A carbonyl group undergoes nucleophilic addition reaction that is whenever nucleophile attacks on carbonyl carbon.
Complete answer:
Let's go step by step reaction for better understanding of the reaction:
Let the
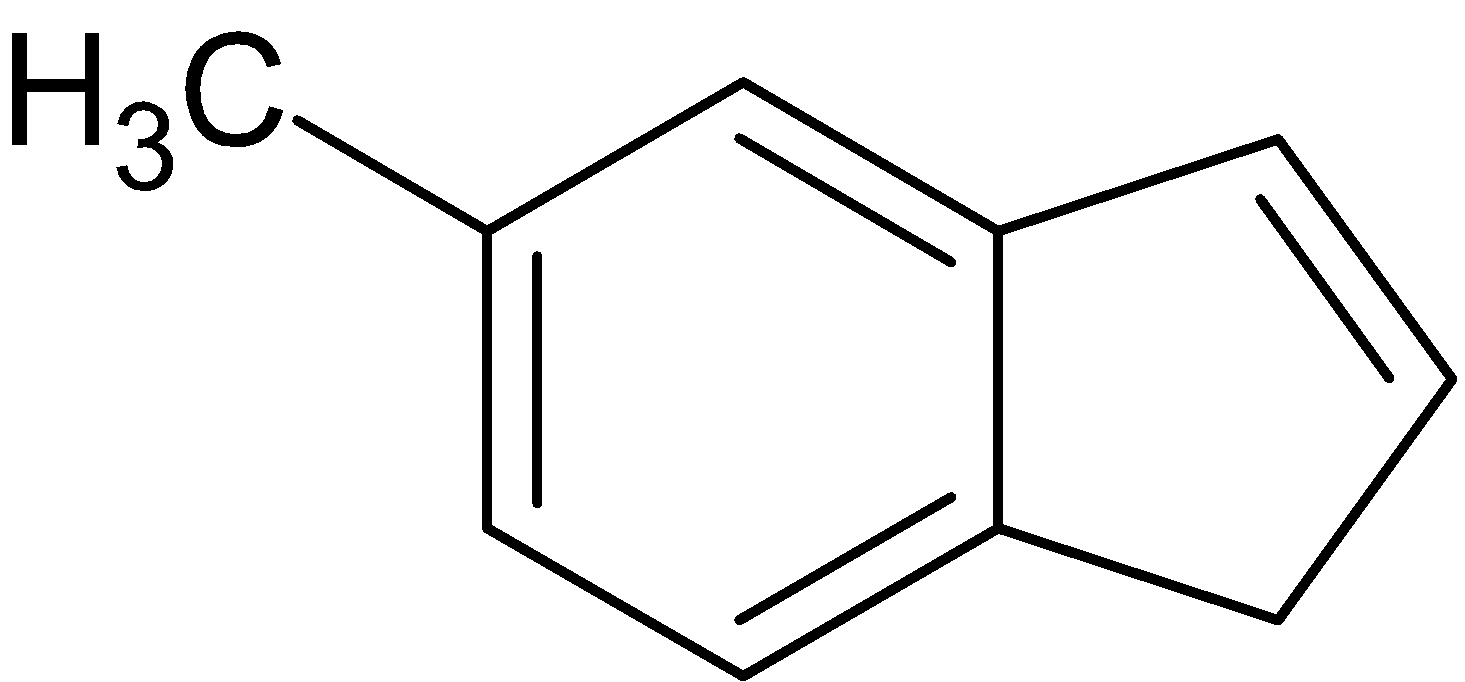 be' H'.
be' H'.
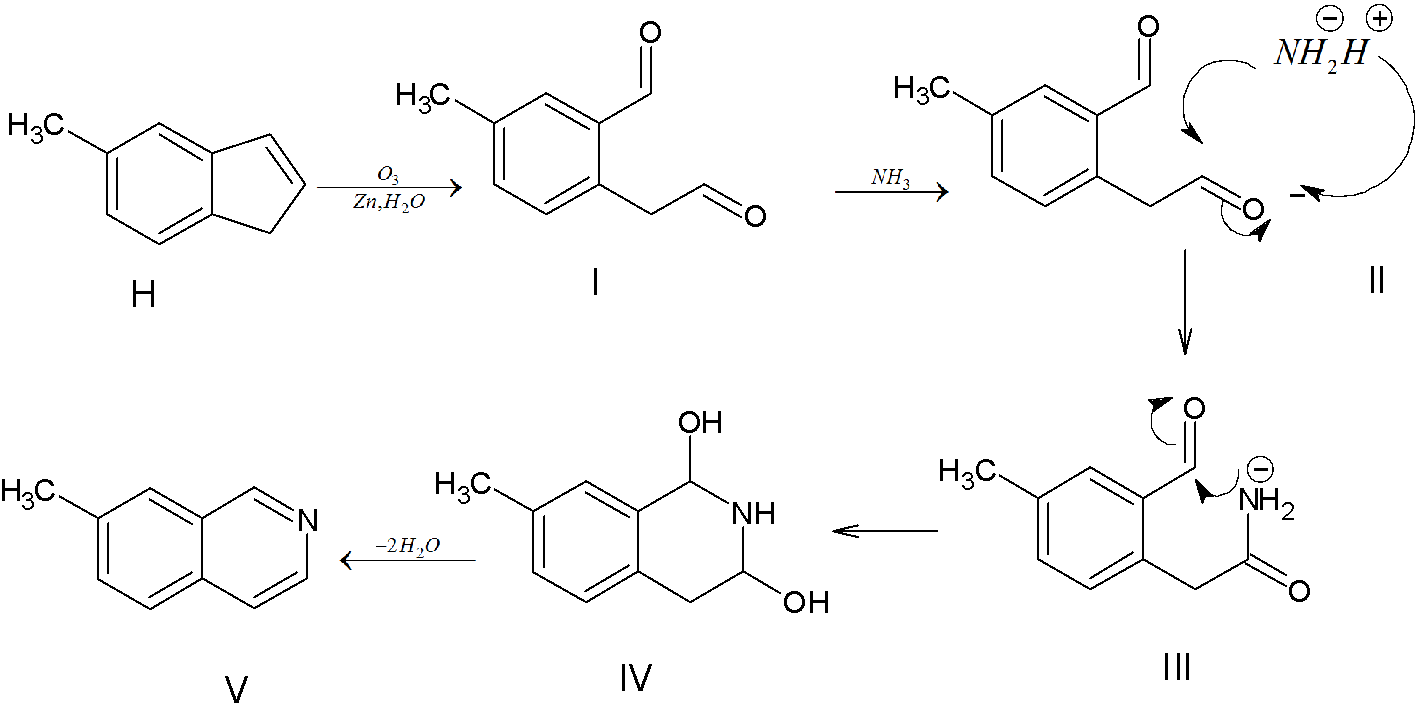
(I) Ozone molecule is added to molecule H that is molecule H undergoes ozonolysis forming corresponding ozonide. The ozonide formed undergoes reductive workup. In the reductive workup process the ozonide is treated with mild reagents like Zn dust in water or dimethyl sulfide resulting in formation of carbonyl compounds. 5-methyl-2-(2oxoethyl)benzaldehyde is formed after the ozonolysis followed by reductive workup.
(II) Addition of ammonia to 5-methyl-2-(2oxoethyl)benzaldehyde. The ammonia molecule can be represented as $NH_2^ - {H^ + }$. The $NH_2^ - $ attack the carbonyl group. When $NH_2^ - $ attacks the positively charged carbonyl carbon to form a new bond. As the new bond is formed, $\pi - bond$ between the carbon and oxygen is broken. The electron pair goes to oxygen, which acquires a negative charge. The ${H^ + }$ (electrophile) attacks negatively charged oxygen to form the addition product (III)
(III) $NH_2^ - $ attacks other carbonyl carbon due to which a new bond is formed, $\pi - bond$ between the carbon and oxygen is broken. The electron pair goes to oxygen, which acquires a negative charge. ${H^ + }$ ion lost by $N{H_2}$, attacks the negatively charged oxygen. Thus (IV) is formed as the addition product.
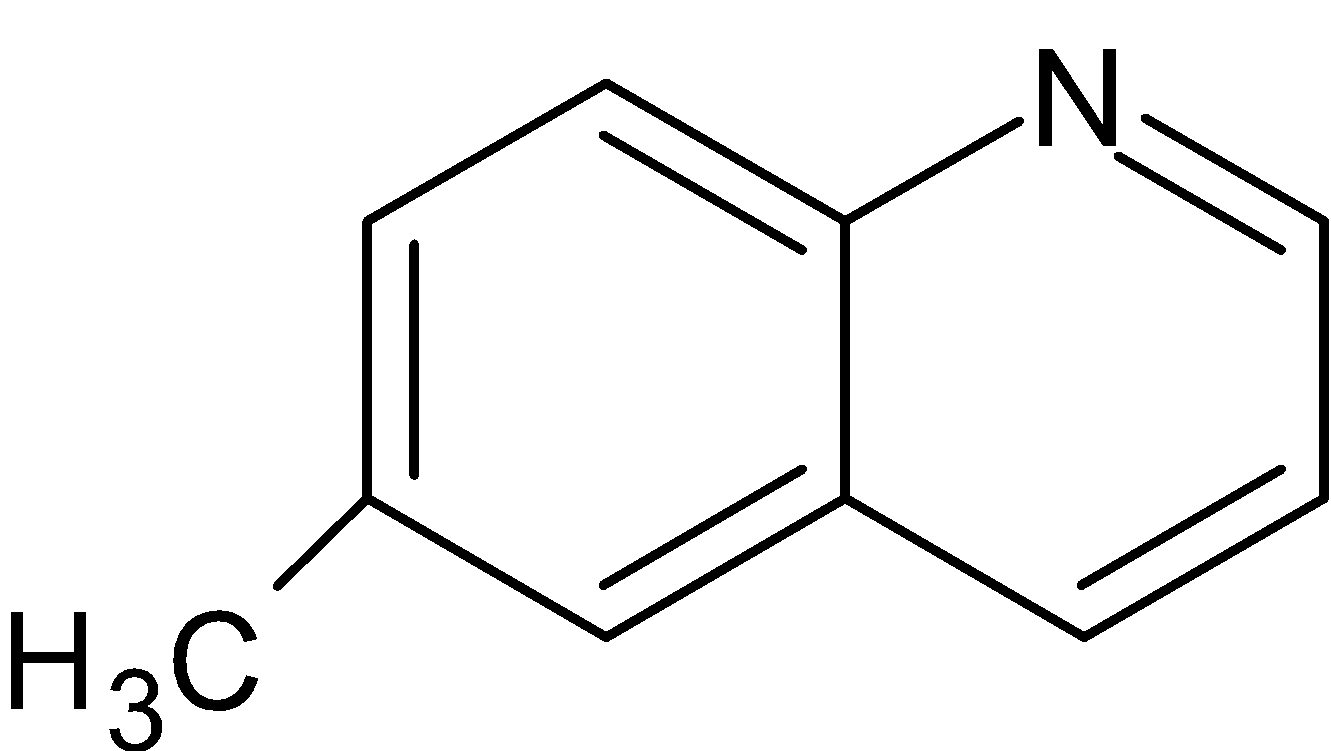
(V) Two molecules of water are removed as a result 7-methylisoquinoline is formed.
Thus, option A is the correct answer.
Note:
The nucleophile attacks the positively charged carbonyl carbon to form a new bond. As the new bond is formed, $\pi - bond$ between the carbon and oxygen is broken. The electron pair goes to oxygen, which acquires a negative charge. The electrophile attacks negatively charged oxygen to form the additional product. This type of reaction is known as nucleophilic addition reaction.
Complete answer:
Let's go step by step reaction for better understanding of the reaction:
Let the

(I) Ozone molecule is added to molecule H that is molecule H undergoes ozonolysis forming corresponding ozonide. The ozonide formed undergoes reductive workup. In the reductive workup process the ozonide is treated with mild reagents like Zn dust in water or dimethyl sulfide resulting in formation of carbonyl compounds. 5-methyl-2-(2oxoethyl)benzaldehyde is formed after the ozonolysis followed by reductive workup.
(II) Addition of ammonia to 5-methyl-2-(2oxoethyl)benzaldehyde. The ammonia molecule can be represented as $NH_2^ - {H^ + }$. The $NH_2^ - $ attack the carbonyl group. When $NH_2^ - $ attacks the positively charged carbonyl carbon to form a new bond. As the new bond is formed, $\pi - bond$ between the carbon and oxygen is broken. The electron pair goes to oxygen, which acquires a negative charge. The ${H^ + }$ (electrophile) attacks negatively charged oxygen to form the addition product (III)
(III) $NH_2^ - $ attacks other carbonyl carbon due to which a new bond is formed, $\pi - bond$ between the carbon and oxygen is broken. The electron pair goes to oxygen, which acquires a negative charge. ${H^ + }$ ion lost by $N{H_2}$, attacks the negatively charged oxygen. Thus (IV) is formed as the addition product.
(V) Two molecules of water are removed as a result 7-methylisoquinoline is formed.

Thus, option A is the correct answer.
Note:
The nucleophile attacks the positively charged carbonyl carbon to form a new bond. As the new bond is formed, $\pi - bond$ between the carbon and oxygen is broken. The electron pair goes to oxygen, which acquires a negative charge. The electrophile attacks negatively charged oxygen to form the additional product. This type of reaction is known as nucleophilic addition reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




