
In the following reaction the product B is
\[A\xrightarrow{Bro\min ation}B\xrightarrow{NaN{{O}_{2}}/HCl}C\xrightarrow[{{C}_{2}}{{H}_{5}}OH]{Boiling}tribromobenzene\]
A. Salicylic acid
B. Benzoic acid
C. Phenol
D. 2,4,6-tribromoaniline
Answer
585k+ views
Hint: In the given question, we have given that there are three sequences of organic reactions. First reaction is a bromination, second one is diazotization and the third one is boiling in presence of ethyl alcohol
Complete step by step answer:
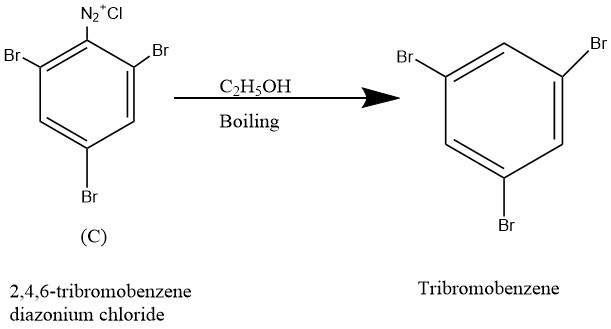
The tribromobenzene can be obtained from diazonium salt by boiling with ethyl alcohol. Thus the compound C could be 2,4,6-tribromobenzene diazonium chloride. The reaction is given below;
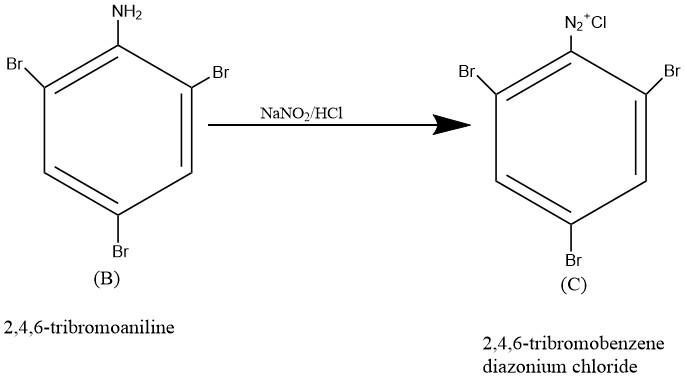
The compound 2, 4, -tribromobenzene diazonium chloride(C) could be prepared by diazotization if \[2,\text{ }4,\text{ }6-tribromoaniline\left( B \right)\] with \[NaN{{O}_{2}}/HCl\] at low temperature. The reaction is given below;
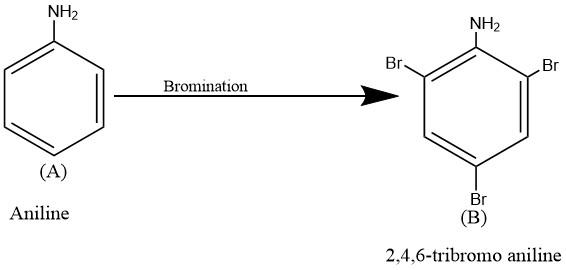
The compound \[2,\text{ }4,\text{ }6-tribromoaniline\] (B) could be obtained by bromination of aniline group. Hence, the compound A could be aniline.the reaction is given below
From these steps we got that the product B is \[2,\text{ }4,\text{ }6-tribromoaniline\]
The complete reaction sequence can be represented as follows
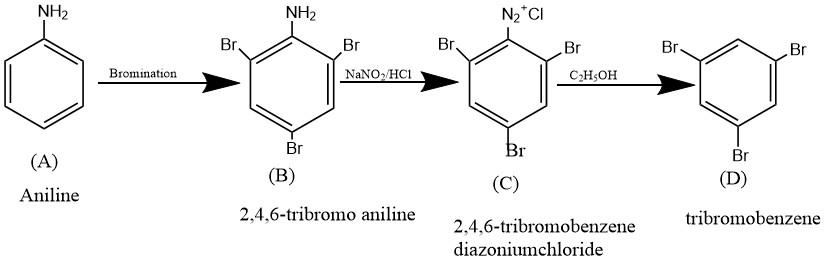
So, the correct answer is Option D.
Note:
Bromination of both phenol and aniline is very difficult to control, with di-, and tri-bromo products forming readily. Because of their high nucleophilic reactivity, aniline phenol undergoes substitution reactions with iodine, a halogen that is normally unreactive with benzene derivatives. In the question the reactions occur in a step by step manner. Starting with bromination of aniline , then diazotization of tribromoaniline and ends with tribromobenzene
In the solution the sequence of reactions starts with aniline. Like aniline there are several benzene derivatives(functional groups attached in benzene) such as phenol, carboxylic acid which gives different products in respective steps.so, we have to be aware of such things
Complete step by step answer:
The tribromobenzene can be obtained from diazonium salt by boiling with ethyl alcohol. Thus the compound C could be 2,4,6-tribromobenzene diazonium chloride. The reaction is given below;

The compound 2, 4, -tribromobenzene diazonium chloride(C) could be prepared by diazotization if \[2,\text{ }4,\text{ }6-tribromoaniline\left( B \right)\] with \[NaN{{O}_{2}}/HCl\] at low temperature. The reaction is given below;

The compound \[2,\text{ }4,\text{ }6-tribromoaniline\] (B) could be obtained by bromination of aniline group. Hence, the compound A could be aniline.the reaction is given below

From these steps we got that the product B is \[2,\text{ }4,\text{ }6-tribromoaniline\]
The complete reaction sequence can be represented as follows

So, the correct answer is Option D.
Note:
Bromination of both phenol and aniline is very difficult to control, with di-, and tri-bromo products forming readily. Because of their high nucleophilic reactivity, aniline phenol undergoes substitution reactions with iodine, a halogen that is normally unreactive with benzene derivatives. In the question the reactions occur in a step by step manner. Starting with bromination of aniline , then diazotization of tribromoaniline and ends with tribromobenzene
In the solution the sequence of reactions starts with aniline. Like aniline there are several benzene derivatives(functional groups attached in benzene) such as phenol, carboxylic acid which gives different products in respective steps.so, we have to be aware of such things
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




