
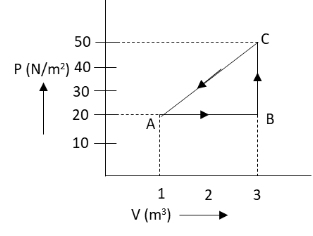
In the diagram, the graph between volume and pressure for a thermodynamic process in shown, if \[{U_A} = 0\], \[{U_B} = 20\,{\text{J}}\] and the energy given from B to C is \[30\,{\text{J}}\], then at stage of C, the internal energy of the system is:
A. \[50\,{\text{J}}\]
B. \[60\,{\text{J}}\]
C. \[30\,{\text{J}}\]
D. \[10\,{\text{J}}\]

Answer
567k+ views
Hint:Use the formula for pressure volume work done by the gas. This formula gives the relation between the pressure of the gas and change in volume of the gas. From this formula equation, determine the work done by the gas from point B to C. Use the formula for heat exchanged by the gas with the surrounding in terms of change in internal energy of the gas. Hence, calculate the internal energy of the gas at stage C.
Formulae used:
The pressure volume work \[W\] done by a gas is given by
\[W = P\Delta V\] …… (1)
Here, \[P\] is pressure of the gas and \[\Delta V\] is change in volume of the gas.
The heat exchanged \[\Delta Q\] by the gas with the surrounding is given by
\[\Delta Q = \Delta U + \Delta W\] …… (2)
Here, \[\Delta U\] is a change in internal energy of the gas and \[\Delta W\] is the change in work done by the gas.
Complete step by step answer:
We have given the graph between the volume and pressure of a gas for a thermodynamic process. The internal energy of the gas at point A and B is \[0\,{\text{J}}\] and \[20\,{\text{J}}\] respectively.
\[{U_A} = 0\,{\text{J}}\]
\[\Rightarrow{U_B} = 20\,{\text{J}}\]
The energy given to the gas from point B to C is \[30\,{\text{J}}\].
\[\Delta Q = 30\,{\text{J}}\]
We have asked to determine the internal energy of the system at stage C. In the given pressure volume graph, we can see that the volume of the gas from point B to C is constant. Hence, the change in volume of the gas from point B to C is zero. Hence, according to equation (1), the pressure volume work done by the gas from point B to C Is zero.
\[\Delta W = 0\,{\text{J}}\]
The change in internal energy of the gas from point B to C is given by
\[\Delta U = {U_C} - {U_B}\]
Let now calculate the internal energy of the gas at point C using equation (2).
Substitute \[{U_C} - {U_B}\] for \[\Delta U\] and \[0\,{\text{J}}\] for \[\Delta W\] in the equation (2).
\[\Delta Q = {U_C} - {U_B} + \left( {0\,{\text{J}}} \right)\]
\[\Rightarrow\Delta Q = {U_C} - {U_B}\]
Rearrange the above equation for \[{U_C}\].
\[{U_C} = \Delta Q + {U_B}\]
Substitute \[30\,{\text{J}}\] for \[\Delta Q\] and \[20\,{\text{J}}\] for \[{U_B}\] in the above equation.
\[{U_C} = \left( {30\,{\text{J}}} \right) + \left( {20\,{\text{J}}} \right)\]
\[ \therefore {U_C} = 50\,{\text{J}}\]
Therefore, the internal energy of the gas at stage C is \[50\,{\text{J}}\].
Hence, the correct option is A.
Note:The students should not get confused between the difference values of the energy given in the question. The energy is given to the system of gas from point B to C. This energy is the energy provided by the surrounding to the system of gas in the thermodynamic process and not the work done by the gas from point B to C or any other energy.
Formulae used:
The pressure volume work \[W\] done by a gas is given by
\[W = P\Delta V\] …… (1)
Here, \[P\] is pressure of the gas and \[\Delta V\] is change in volume of the gas.
The heat exchanged \[\Delta Q\] by the gas with the surrounding is given by
\[\Delta Q = \Delta U + \Delta W\] …… (2)
Here, \[\Delta U\] is a change in internal energy of the gas and \[\Delta W\] is the change in work done by the gas.
Complete step by step answer:
We have given the graph between the volume and pressure of a gas for a thermodynamic process. The internal energy of the gas at point A and B is \[0\,{\text{J}}\] and \[20\,{\text{J}}\] respectively.
\[{U_A} = 0\,{\text{J}}\]
\[\Rightarrow{U_B} = 20\,{\text{J}}\]
The energy given to the gas from point B to C is \[30\,{\text{J}}\].
\[\Delta Q = 30\,{\text{J}}\]
We have asked to determine the internal energy of the system at stage C. In the given pressure volume graph, we can see that the volume of the gas from point B to C is constant. Hence, the change in volume of the gas from point B to C is zero. Hence, according to equation (1), the pressure volume work done by the gas from point B to C Is zero.
\[\Delta W = 0\,{\text{J}}\]
The change in internal energy of the gas from point B to C is given by
\[\Delta U = {U_C} - {U_B}\]
Let now calculate the internal energy of the gas at point C using equation (2).
Substitute \[{U_C} - {U_B}\] for \[\Delta U\] and \[0\,{\text{J}}\] for \[\Delta W\] in the equation (2).
\[\Delta Q = {U_C} - {U_B} + \left( {0\,{\text{J}}} \right)\]
\[\Rightarrow\Delta Q = {U_C} - {U_B}\]
Rearrange the above equation for \[{U_C}\].
\[{U_C} = \Delta Q + {U_B}\]
Substitute \[30\,{\text{J}}\] for \[\Delta Q\] and \[20\,{\text{J}}\] for \[{U_B}\] in the above equation.
\[{U_C} = \left( {30\,{\text{J}}} \right) + \left( {20\,{\text{J}}} \right)\]
\[ \therefore {U_C} = 50\,{\text{J}}\]
Therefore, the internal energy of the gas at stage C is \[50\,{\text{J}}\].
Hence, the correct option is A.
Note:The students should not get confused between the difference values of the energy given in the question. The energy is given to the system of gas from point B to C. This energy is the energy provided by the surrounding to the system of gas in the thermodynamic process and not the work done by the gas from point B to C or any other energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




