
In sodium dihydrogen pyrophosphate, the number of non-acidic hydrogen is ‘x’, the number of oxygen atoms is 'y', and the oxidation state of each phosphorus atom is 'z', then the value of (x+y-z) is:
(a)- 4
(b)- 6
(c)- 0
(d)- 2
Answer
529.5k+ views
Hint: Sodium hydrogen pyro phosphite is an inorganic compound in which the elements present are oxygen, hydrogen, sodium, and phosphorus, so its formula is $N{{a}_{2}}{{H}_{2}}{{P}_{2}}{{O}_{7}}$. If the hydrogen atom is not attached to an electronegative atom then it is a non-acidic hydrogen atom.
Complete answer:
Disodium dihydrogen diphosphate is an IUPAC name of Sodium dihydrogen pyrophosphate which is an inorganic compound in which the elements present are oxygen, hydrogen, sodium, and phosphorus. The formula of Sodium hydrogen pyro phosphite is $N{{a}_{2}}{{H}_{2}}{{P}_{2}}{{O}_{7}}$.
Let us see the structure of the compound.
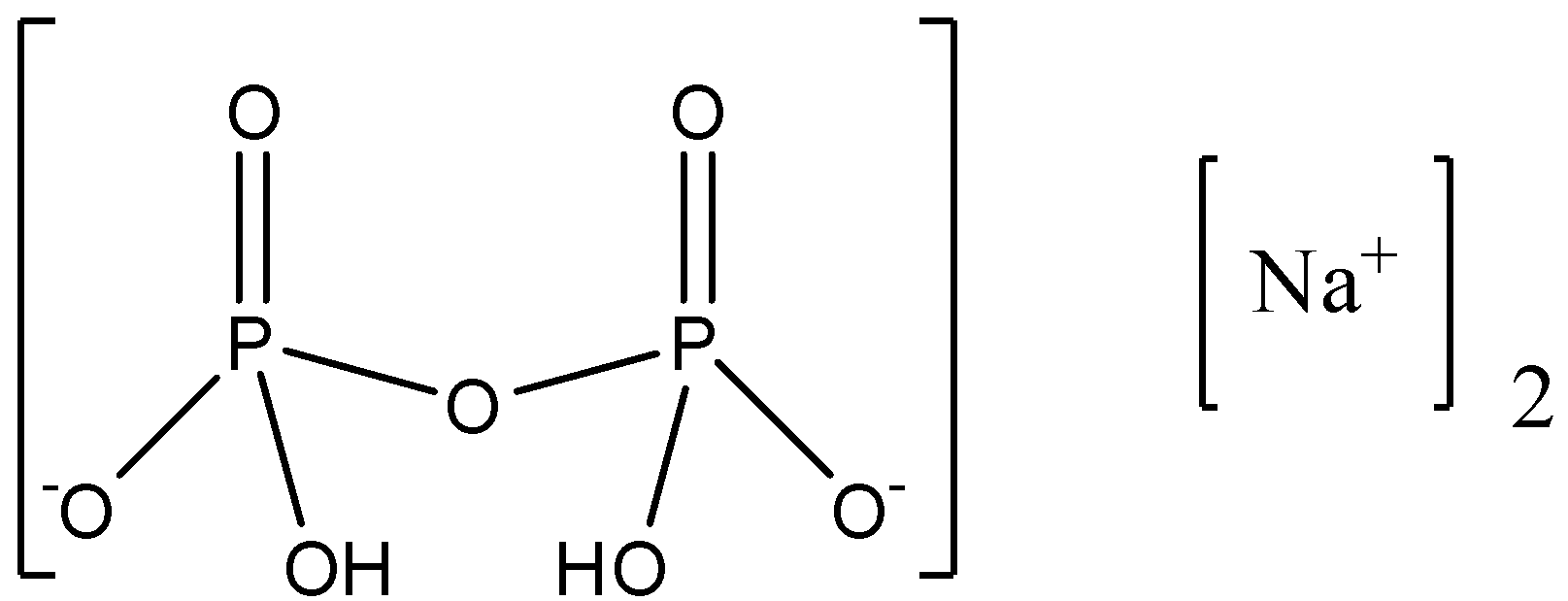
If the hydrogen atom is not attached to an electronegative atom then it is a non-acidic hydrogen atom. As we can see that there are two hydrogen atoms in the compound and both the hydrogen atoms are attached to the oxygen atom, an oxygen atom is electronegative, so both the hydrogen atoms are acidic hydrogen.
Therefore, the number of non-acidic hydrogen ‘x’ is 0.
From the formula we can see that the number of oxygen atoms are 7, therefore, ‘y ‘ is 7.
Now, we have to find the oxidation number of phosphorus in the compound. The oxidation state is calculated as follows:
$N{{a}_{2}}{{H}_{2}}{{P}_{2}}{{O}_{7}}$
$2\text{ x (+1) + 2 x}\text{ (+1) + 2}x\text{ + 7 x (-2) = 0}$
$\text{2 + 2 + 2}x\text{ - 14 = 0}$
$x=+5$
Therefore, the value of ‘z’ is +5.
Now, we have to find the value of (x+y-z), so by putting the values, we get:
$(x+y-z)=(0+7-(+5))$
$(x+y-z)=7-5=2$
So, the answer is 2.
Therefore, the correct answer is an option (d).
Note:
In the compound, if the hydrogen atom was directly attached to the phosphorus atom then, it would be counted as a non-acidic hydrogen atom. While calculating the oxidation state, the oxidation number of sodium is taken +1 because it is an alkali metal
Complete answer:
Disodium dihydrogen diphosphate is an IUPAC name of Sodium dihydrogen pyrophosphate which is an inorganic compound in which the elements present are oxygen, hydrogen, sodium, and phosphorus. The formula of Sodium hydrogen pyro phosphite is $N{{a}_{2}}{{H}_{2}}{{P}_{2}}{{O}_{7}}$.
Let us see the structure of the compound.

If the hydrogen atom is not attached to an electronegative atom then it is a non-acidic hydrogen atom. As we can see that there are two hydrogen atoms in the compound and both the hydrogen atoms are attached to the oxygen atom, an oxygen atom is electronegative, so both the hydrogen atoms are acidic hydrogen.
Therefore, the number of non-acidic hydrogen ‘x’ is 0.
From the formula we can see that the number of oxygen atoms are 7, therefore, ‘y ‘ is 7.
Now, we have to find the oxidation number of phosphorus in the compound. The oxidation state is calculated as follows:
$N{{a}_{2}}{{H}_{2}}{{P}_{2}}{{O}_{7}}$
$2\text{ x (+1) + 2 x}\text{ (+1) + 2}x\text{ + 7 x (-2) = 0}$
$\text{2 + 2 + 2}x\text{ - 14 = 0}$
$x=+5$
Therefore, the value of ‘z’ is +5.
Now, we have to find the value of (x+y-z), so by putting the values, we get:
$(x+y-z)=(0+7-(+5))$
$(x+y-z)=7-5=2$
So, the answer is 2.
Therefore, the correct answer is an option (d).
Note:
In the compound, if the hydrogen atom was directly attached to the phosphorus atom then, it would be counted as a non-acidic hydrogen atom. While calculating the oxidation state, the oxidation number of sodium is taken +1 because it is an alkali metal
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




