
In $S{N_2}$ solvolysis of $RX$ in which the solvent is a nucleophile, what is the order and molecularity of the reaction?
A. First order; unimolecular
B. First order; bimolecular
C. Second order; bimolecular
D. Pseudo first-order; bimolecular
Answer
595.5k+ views
Hint: As the name suggests, $S{N_2}$ stands for bimolecular substitution reaction which is a bimolecular reaction. There is an immediate attack of nucleophile from the rear side and the leaving group leaves the transition state from the front side. In case of solvolysis, the concentration of the solvent is considered negligible as the concentration of solute is too high as compared to the concentration of the solvent.
Complete answer:
The solvolysis of an alkyl halide produces the following reaction:
$R - X + solvent(N{u^ - })\xrightarrow{{solvolysis}}R - Nu + {X^ - }$
In this reaction, there is a formation of unstable intermediates. The reaction mechanism includes the attack of the nucleophile from the rear side of the alkyl group and the halide group leaves from the front side of the alkyl compound.
The unstable reaction intermediate formed looks like:
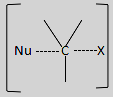
In this, there is a simultaneous breaking of old bonds and formation of new bonds.
Now, coming to the solvolysis reaction, the rate law is written as:
$Rate = k[RX][solvent]$
As the concentration of the solute,$RX$, is very less as compared to the concentration of the solvent or conversely saying, the solvent is too dilute, the concentration of the solvent is considered unity and is ignored while calculating the rate law of the reaction.
So, the correct answer is Option D.
Note:
Although the molecularity of the pseudo-bimolecular reaction is 2, due to the presence of one of the molecules in excess, its concentration is considered unity and the reaction becomes pseudo.
Complete answer:
The solvolysis of an alkyl halide produces the following reaction:
$R - X + solvent(N{u^ - })\xrightarrow{{solvolysis}}R - Nu + {X^ - }$
In this reaction, there is a formation of unstable intermediates. The reaction mechanism includes the attack of the nucleophile from the rear side of the alkyl group and the halide group leaves from the front side of the alkyl compound.
The unstable reaction intermediate formed looks like:

In this, there is a simultaneous breaking of old bonds and formation of new bonds.
Now, coming to the solvolysis reaction, the rate law is written as:
$Rate = k[RX][solvent]$
As the concentration of the solute,$RX$, is very less as compared to the concentration of the solvent or conversely saying, the solvent is too dilute, the concentration of the solvent is considered unity and is ignored while calculating the rate law of the reaction.
So, the correct answer is Option D.
Note:
Although the molecularity of the pseudo-bimolecular reaction is 2, due to the presence of one of the molecules in excess, its concentration is considered unity and the reaction becomes pseudo.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




