
In $ (S{i_2}{O_5})_n^{2n - } $ anion is obtained when:
(A) No oxygen of a $ SiO_4^{4 - } $ tetrahedron is shared with another $ SiO_4^{4 - } $ tetrahedron.
(B) one oxygen of a $ SiO_4^{4 - } $ tetrahedron is shared with another $ SiO_4^{4 - } $ tetrahedron.
(C) two oxygen of a $ SiO_4^{4 - } $ tetrahedron is shared with another $ SiO_4^{4 - } $ tetrahedron.
(D) three oxygen of a $ SiO_4^{4 - } $ tetrahedron is shared with another $ SiO_4^{4 - } $ tetrahedron.
Answer
547.5k+ views
Hint: We need the concept of silicates here. Also, the different types of silicates should be known. It is a part of inorganic chemistry. Depending on the type of bonds, there are seven silicate types.
Complete step by step answer:
We already know the formula: $ (S{i_2}{O_5})_n^{2n - } $ .
Silicates are the minerals containing silicon and oxygen in tetrahedral $ SiO_4^{4 - } $ units, which are linked together in several patterns. Depending on the way the tetrahedral units are linked, the silicates are classified into the seven types.
The following structure is given below:
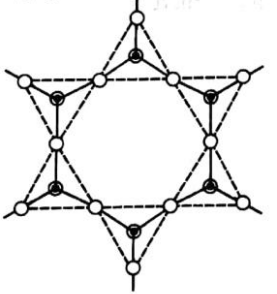
The general formula of Sheet or Phyllo or two dimensional (2-D) silicates is $ (S{i_2}{O_5})_n^{2n - } $ . Each $ Si{O_4} $ tetrahedron shares three oxygen atoms with others and thus by forming two-dimensional sheets. These silicates can be cleaved easily just like graphite. The layers are held together by weak van der Waals forces.
Total no. of oxygen atoms per silicon atom = $ \dfrac{1}{2} + \dfrac{1}{2} + \dfrac{1}{2} + 1 = \dfrac{5}{2} = 2.5 $
So, the formula comes out to be $ S{i_2}{O_5}^{ - 2} $
Now we need to select the correct option.
Thus, the correct option is D.
Note:
Silicate minerals are very common in the Earth crust since Oxygen and Silicon are the most abundant elements. The degree of polymerization is denoted by Oxygen to Silicon ratio (O/Si). Greater the degree of polymerization, lower will be the O/Si ratio. With increase in the degree of polymerization, there is decrease in the charge per silicon atom as well as the basicity of silicate minerals. The basic silicate minerals readily react with weak acids and undergo weathering.
Some examples of sheet or Phyllo or two dimensional (2-D) silicates are talc and micas. Talc is the main ingredient of soap stone. Itis the softest material with a smooth and greasy touch.
Complete step by step answer:
We already know the formula: $ (S{i_2}{O_5})_n^{2n - } $ .
Silicates are the minerals containing silicon and oxygen in tetrahedral $ SiO_4^{4 - } $ units, which are linked together in several patterns. Depending on the way the tetrahedral units are linked, the silicates are classified into the seven types.
The following structure is given below:

The general formula of Sheet or Phyllo or two dimensional (2-D) silicates is $ (S{i_2}{O_5})_n^{2n - } $ . Each $ Si{O_4} $ tetrahedron shares three oxygen atoms with others and thus by forming two-dimensional sheets. These silicates can be cleaved easily just like graphite. The layers are held together by weak van der Waals forces.
Total no. of oxygen atoms per silicon atom = $ \dfrac{1}{2} + \dfrac{1}{2} + \dfrac{1}{2} + 1 = \dfrac{5}{2} = 2.5 $
So, the formula comes out to be $ S{i_2}{O_5}^{ - 2} $
Now we need to select the correct option.
Thus, the correct option is D.
Note:
Silicate minerals are very common in the Earth crust since Oxygen and Silicon are the most abundant elements. The degree of polymerization is denoted by Oxygen to Silicon ratio (O/Si). Greater the degree of polymerization, lower will be the O/Si ratio. With increase in the degree of polymerization, there is decrease in the charge per silicon atom as well as the basicity of silicate minerals. The basic silicate minerals readily react with weak acids and undergo weathering.
Some examples of sheet or Phyllo or two dimensional (2-D) silicates are talc and micas. Talc is the main ingredient of soap stone. Itis the softest material with a smooth and greasy touch.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




