
In Rutile $Ti{{O}_{2}}$ structure, if the number of an effective number of molecules in each unit cell is x and the coordination number of oxygen in the unit cell is y. What is the value of x+y.
In the figure given about red balls represents oxygen atoms and white balls represents titanium atoms:
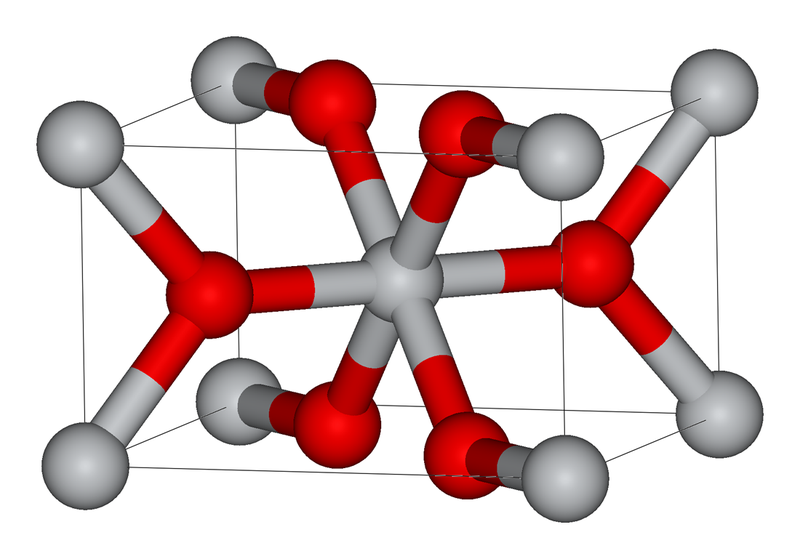
A.4
B.6
C.5
D.8

Answer
537k+ views
Hint: Before answering this question, we should first know about Rutile. Titanium oxide ($Ti{{O}_{2}}$) is a mineral which is also known as Rutile. Its refractive indices are one of the highest at visible wavelengths of any crustal. It has high dispersion and large birefringence.
Complete answer:
The name Rutile is derived from the Latin word Rutilus(Red) as the deep red color could be observed in some species if we see it through transmitted light. Abraham Gottlob Werner discovered Rutile in 1803. The composition of natural rutile is there is 10% of iron and Niobium and tantalum.
In High temperature and pressure metamorphic rocks and igneous rocks, Rutile is a usual mineral that is used. It is considered one of the most stable polymorphs $Ti{{O}_{2}}$ at every temperature.
It has a tetragonal unit cell and has a framework of unit cell i.e a = b = 4.584 ${{A}^{\circ }}$and c = 2.953 ${{A}^{\circ }}$. The positive ions of titanium have coordination number six, which means they are enclosed by octahedron having six oxygen atoms and the coordination number of negative ions of oxygen is three i.e trigonal planar coordination.
Titanium atoms in titanium oxide form a bcc arrangement. This unit cell consists of two atoms per unit cell. So, x = 2
Oxygen atoms are in titanium oxide are in trigonal voids. This void’s coordination number is three. So, y = 3
Therefore, x+y = 2+3 = 5
So, Option (C) 5 is correct.
Note:
Rutile is a vital part of ore deposits and heavy minerals. Miners help in the extraction and separation of useful and valuable minerals like Zircon, ilmenite. Some of its uses are- As a pigment, in the manufacture of titanium metal, refractory ceramic.
Complete answer:
The name Rutile is derived from the Latin word Rutilus(Red) as the deep red color could be observed in some species if we see it through transmitted light. Abraham Gottlob Werner discovered Rutile in 1803. The composition of natural rutile is there is 10% of iron and Niobium and tantalum.
In High temperature and pressure metamorphic rocks and igneous rocks, Rutile is a usual mineral that is used. It is considered one of the most stable polymorphs $Ti{{O}_{2}}$ at every temperature.
It has a tetragonal unit cell and has a framework of unit cell i.e a = b = 4.584 ${{A}^{\circ }}$and c = 2.953 ${{A}^{\circ }}$. The positive ions of titanium have coordination number six, which means they are enclosed by octahedron having six oxygen atoms and the coordination number of negative ions of oxygen is three i.e trigonal planar coordination.
Titanium atoms in titanium oxide form a bcc arrangement. This unit cell consists of two atoms per unit cell. So, x = 2
Oxygen atoms are in titanium oxide are in trigonal voids. This void’s coordination number is three. So, y = 3
Therefore, x+y = 2+3 = 5
So, Option (C) 5 is correct.
Note:
Rutile is a vital part of ore deposits and heavy minerals. Miners help in the extraction and separation of useful and valuable minerals like Zircon, ilmenite. Some of its uses are- As a pigment, in the manufacture of titanium metal, refractory ceramic.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE

Differentiate between calcination and roasting class 11 chemistry CBSE




