
In preparation of p-nitroacetanilide another mirror product is formed. What is the compound and how can this be separated from p-nitroacetanilide?
Answer
509.1k+ views
Hint: The p-nitroacetanilide is an organic compound. Its preparation is carried out through the process of Nitration. It is also called the \[4 - nitroace\tan ilide\]. In this reaction a side product is formed also known as mirror product.
Complete answer:
Let’s see the reaction involved in the preparation of the p-nitroacetanilide
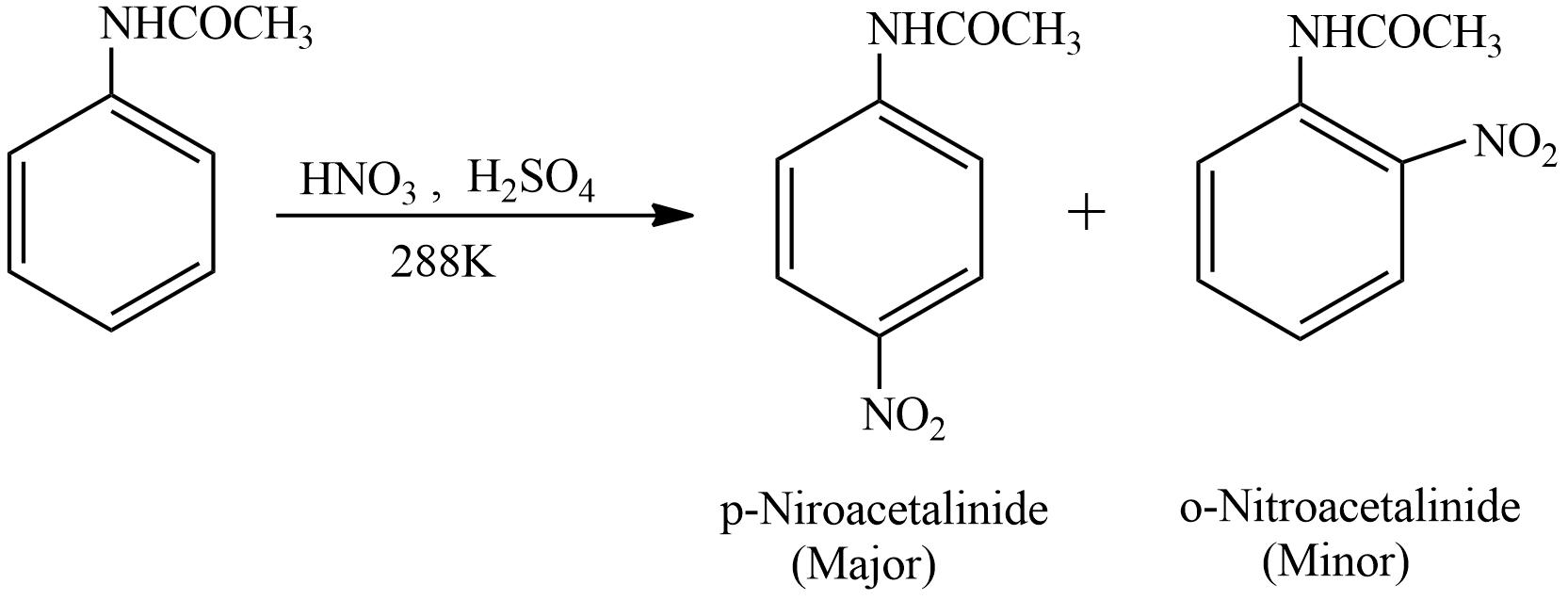
This is an electrophilic substitution reaction. Acetanilide undergoes a Nitration process in presence of Nitric and Sulphuric acid at a temperature of \[288K\]. Mixture of Nitric acid and sulphuric acid is known as Nitrating Mixture. The products formed are p-nitroacetanilide and o-nitroacetanilide. The nitro group will be attached to the para position because \[NHCOC{H_3}\] is an electron releasing group. The product para product is major product (more quantity) and ortho product is minor product (less quantity).
The minor product that is o-nitroacetanilide is separated from the p-nitroacetanilide by dissolving the solution of both products in Ethanol. o-nitroacetanilide is more soluble in alcohol as compared to the p-nitroacetanilide. During Crystallization process the o-nitroacetanilide remains in the solution as it is soluble in alcohol and p-nitroacetanilide crystallises. Then crystals of the p-nitroacetanilide are easily filtered with the help of a filter paper from the solution. The crystals of the p-nitroacetanilide are colourless.
Note:
Nitro anilines can be prepared from the product formed in this reaction as direct nitration of aniline is not possible because amino groups are oxidised when treated with nitrating mixture. For preparing nitroaniline acetanilide is subjected to nitration to form p-nitroacetanilide. Then p-acetanilide is subjected to hydrolysis to obtain p-nitroaniline.
Complete answer:
Let’s see the reaction involved in the preparation of the p-nitroacetanilide

This is an electrophilic substitution reaction. Acetanilide undergoes a Nitration process in presence of Nitric and Sulphuric acid at a temperature of \[288K\]. Mixture of Nitric acid and sulphuric acid is known as Nitrating Mixture. The products formed are p-nitroacetanilide and o-nitroacetanilide. The nitro group will be attached to the para position because \[NHCOC{H_3}\] is an electron releasing group. The product para product is major product (more quantity) and ortho product is minor product (less quantity).
The minor product that is o-nitroacetanilide is separated from the p-nitroacetanilide by dissolving the solution of both products in Ethanol. o-nitroacetanilide is more soluble in alcohol as compared to the p-nitroacetanilide. During Crystallization process the o-nitroacetanilide remains in the solution as it is soluble in alcohol and p-nitroacetanilide crystallises. Then crystals of the p-nitroacetanilide are easily filtered with the help of a filter paper from the solution. The crystals of the p-nitroacetanilide are colourless.
Note:
Nitro anilines can be prepared from the product formed in this reaction as direct nitration of aniline is not possible because amino groups are oxidised when treated with nitrating mixture. For preparing nitroaniline acetanilide is subjected to nitration to form p-nitroacetanilide. Then p-acetanilide is subjected to hydrolysis to obtain p-nitroaniline.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




