
In ${{\text{P}}_{4}}{{\text{O}}_{10}}$, the number of oxygen atoms attached to each phosphorus atom is:
A. 2
B. 3
C. 4
D. 5
Answer
593.7k+ views
Hint: ${{\text{P}}_{4}}{{\text{O}}_{10}}$ is a molecular formula of pentoxide which consists of 4 molecules of phosphorus and 10 molecules of oxygen. The phosphorus can make a maximum of 5 bonds with other molecules whereas oxygen can make a maximum of 2 bonds with other molecules.
Complete Answer:
-To count the number of bonds between oxygen and phosphorus we first have to draw the structure of phosphorus pentoxide.
-The structure is given below:
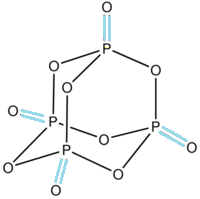
-As we can see in the structure of phosphorus pentoxide there are a total 4 oxygens which are highlighted by blue colour and make double bonds only with a single phosphorus atom.
-The double-bonded oxygen has a hybridisation of $\text{s}{{\text{p}}^{2}}$
-Whereas 6 oxygen atoms are attached to the two phosphorus atoms each with a single bond in which the hybridisation of oxygen atom is $\text{s}{{\text{p}}^{3}}$.
So, option C. is the correct answer.
-The structure of phosphorus pentoxide forms a hexagonal lattice and is held together by the Vander Waal forces.
-The structure consists of at least four polymorphs.
Note: The preparation of phosphorus pentoxide is done when tetraphosphorus reacts with oxygen molecules: ${{\text{P}}_{4}}\text{ + 5}{{\text{O}}_{2}}\text{ }\to \text{ }{{\text{P}}_{4}}{{\text{O}}_{10}}$. The compound is used as a dehydrating agent and is white in appearance. The name of the compound is named so because it is the empirical formula of ${{\text{P}}_{4}}{{\text{O}}_{5}}$.
Complete Answer:
-To count the number of bonds between oxygen and phosphorus we first have to draw the structure of phosphorus pentoxide.
-The structure is given below:

-As we can see in the structure of phosphorus pentoxide there are a total 4 oxygens which are highlighted by blue colour and make double bonds only with a single phosphorus atom.
-The double-bonded oxygen has a hybridisation of $\text{s}{{\text{p}}^{2}}$
-Whereas 6 oxygen atoms are attached to the two phosphorus atoms each with a single bond in which the hybridisation of oxygen atom is $\text{s}{{\text{p}}^{3}}$.
So, option C. is the correct answer.
-The structure of phosphorus pentoxide forms a hexagonal lattice and is held together by the Vander Waal forces.
-The structure consists of at least four polymorphs.
Note: The preparation of phosphorus pentoxide is done when tetraphosphorus reacts with oxygen molecules: ${{\text{P}}_{4}}\text{ + 5}{{\text{O}}_{2}}\text{ }\to \text{ }{{\text{P}}_{4}}{{\text{O}}_{10}}$. The compound is used as a dehydrating agent and is white in appearance. The name of the compound is named so because it is the empirical formula of ${{\text{P}}_{4}}{{\text{O}}_{5}}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




