
In ${{P}_{4}}{{O}_{10}}$ molecule, bridging P-O bond length is:
A. Larger than that of in ${{P}_{4}}{{O}_{6}}$
B. lesser than that of in ${{P}_{4}}{{O}_{6}}$
C. equal to that of in ${{P}_{4}}{{O}_{6}}$
D. cannot be compared
Answer
532.5k+ views
Hint: The presence of double and single bonds leads to the bond length and orientation of bonds in a molecule. ${{P}_{4}}{{O}_{10}} $is tetra phosphorus decaoxide. This molecule consists of a double bond character in the oxygen bond.
Complete answer:
We have to find about the P-O bond length in ${{P}_{4}}{{O}_{10}}$ molecule and compare it with the P-O bond in ${{P}_{4}}{{O}_{6}}$ molecule.
As we know that the multiplicity of bonds affects the bond length. Double bonds and triple bonds tend to be of lesser length than single bonds. This is the reason why some bonds have more bond length and some bonds have less bond length. The presence of any double bond in the oxygen atom in both the oxides of phosphorus will tell us the information about the P-O bridge.
The molecule of ${{P}_{4}}{{O}_{10}}$ is as follows:
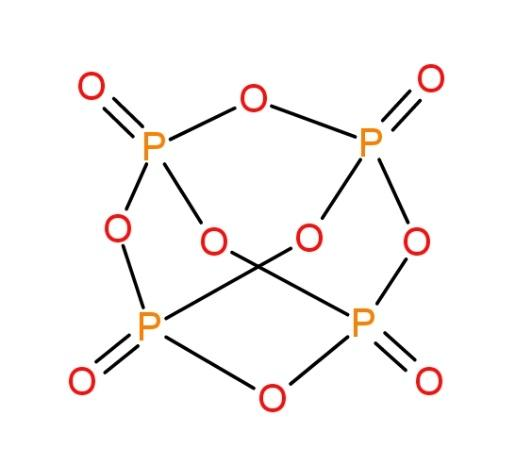
We can see the presence of 4 P-O bonds here. These bonds are double bonds (4) and others single bonds.
Now here is the ${{P}_{4}}{{O}_{6}}$ molecule:
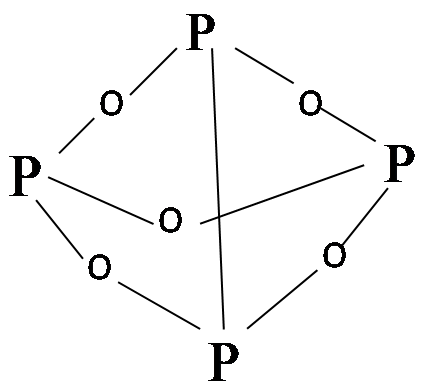
This molecule consists of a network of only single bonds in the P-O bonds.
Clearly, from the nature of P-O bonds present in both the molecules, it can be inferred that P-O bond length in ${{P}_{4}}{{O}_{10}}$ is lesser than that of in ${{P}_{4}}{{O}_{6}}$ molecule, due to the presence of double bonds.
Hence, P-O bond in ${{P}_{4}}{{O}_{10}}$ is lesser than P-O bond in ${{P}_{4}}{{O}_{6}}$.
Thus option B is correct.
Note:
From the observed measurements of the P-O bonds in ${{P}_{4}}{{O}_{6}}$ and ${{P}_{4}}{{O}_{10}}$, the value of P-O bond in ${{P}_{4}}{{O}_{6}}$ is 166 pm, while that of this bond in ${{P}_{4}}{{O}_{10}}$ is 143 pm. This also proves that P-O bond in ${{P}_{4}}{{O}_{6}}$ is larger than P-O bond in ${{P}_{4}}{{O}_{10}}$.
Complete answer:
We have to find about the P-O bond length in ${{P}_{4}}{{O}_{10}}$ molecule and compare it with the P-O bond in ${{P}_{4}}{{O}_{6}}$ molecule.
As we know that the multiplicity of bonds affects the bond length. Double bonds and triple bonds tend to be of lesser length than single bonds. This is the reason why some bonds have more bond length and some bonds have less bond length. The presence of any double bond in the oxygen atom in both the oxides of phosphorus will tell us the information about the P-O bridge.
The molecule of ${{P}_{4}}{{O}_{10}}$ is as follows:

We can see the presence of 4 P-O bonds here. These bonds are double bonds (4) and others single bonds.
Now here is the ${{P}_{4}}{{O}_{6}}$ molecule:

This molecule consists of a network of only single bonds in the P-O bonds.
Clearly, from the nature of P-O bonds present in both the molecules, it can be inferred that P-O bond length in ${{P}_{4}}{{O}_{10}}$ is lesser than that of in ${{P}_{4}}{{O}_{6}}$ molecule, due to the presence of double bonds.
Hence, P-O bond in ${{P}_{4}}{{O}_{10}}$ is lesser than P-O bond in ${{P}_{4}}{{O}_{6}}$.
Thus option B is correct.
Note:
From the observed measurements of the P-O bonds in ${{P}_{4}}{{O}_{6}}$ and ${{P}_{4}}{{O}_{10}}$, the value of P-O bond in ${{P}_{4}}{{O}_{6}}$ is 166 pm, while that of this bond in ${{P}_{4}}{{O}_{10}}$ is 143 pm. This also proves that P-O bond in ${{P}_{4}}{{O}_{6}}$ is larger than P-O bond in ${{P}_{4}}{{O}_{10}}$.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




