
In NaCl structure :
Options are-
(A) all octahedral and tetrahedral sites are occupied
(B) only octahedral sites are occupied
(C) only tetrahedral sites are occupied
(D) neither octahedral or tetrahedral sites are occupied
Answer
577.2k+ views
Hint: An attempt to this question can be made by recalling the structure of NaCl. Determine the position of sodium ions and chlorine ions. Based on this you can determine the ions present in voids and the composition of the voids as well. Based on this you can determine the correct option and answer the question.
Complete answer:
We will determine the structure of NaCl crystals as suggested in the hint.
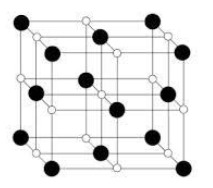
The black atoms correspond to ${Cl^-}$ ions and white atoms correspond to ${Na^+}$ atoms.
In the structure we see that chlorine ions are present in all the corners. On the other hand, sodium ions occupy the octahedral voids. In the structure we see that all of the octahedral voids are occupied.
From the above statements we can conclude that in In NaCl structure only octahedral sites are occupied.
Therefore, the correct answer is option (B).
Note:
It should be remembered to you that on application of high pressure, The NaCl structure changes to CsCl structure whereas on heating the CsCl structure at 760K, the CsCl structure transforms to NaCl structure.
The structure of CsCl is given below:
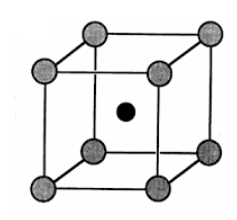
The grey atoms correspond to ${Cl^-}$ ions and black atom is ${Cs^+}$ ion.
Also, you should remember that CsCl is used in isopycnic centrifugation for separating various types of DNA.
Complete answer:
We will determine the structure of NaCl crystals as suggested in the hint.

The black atoms correspond to ${Cl^-}$ ions and white atoms correspond to ${Na^+}$ atoms.
In the structure we see that chlorine ions are present in all the corners. On the other hand, sodium ions occupy the octahedral voids. In the structure we see that all of the octahedral voids are occupied.
From the above statements we can conclude that in In NaCl structure only octahedral sites are occupied.
Therefore, the correct answer is option (B).
Note:
It should be remembered to you that on application of high pressure, The NaCl structure changes to CsCl structure whereas on heating the CsCl structure at 760K, the CsCl structure transforms to NaCl structure.
The structure of CsCl is given below:

The grey atoms correspond to ${Cl^-}$ ions and black atom is ${Cs^+}$ ion.
Also, you should remember that CsCl is used in isopycnic centrifugation for separating various types of DNA.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




