
In $\beta $ - emission from a radioactive substance, an electron is ejected from:
A.The outermost orbit of atom
B.The inner orbits of atom
C.The surface of substance
D.The nucleus of atom
Answer
589.5k+ views
Hint: When there is a change within the nucleus of an atom, emission of particles or electromagnetic radiation takes place. This is radioactive decay. There are different types of emissions from a radioactive substance and are alpha emission ( $\alpha $ ), beta emission ($\beta $), and gamma emission ( $\gamma $).
Complete step by step answer:
As we know that a beta particle is also called beta ray or radiation. It is a high energy and high-speed electron or positron that is emitted by the radioactive decay of the atomic nucleus.
There are 2 types of beta decay, one in which electrons are produced and are ${\beta ^ - }$ decay, and one in which positrons are emitted is ${\beta ^ + }$ decay.
The radioactive atom has an unstable nucleus, hence when radiations are emitted, it becomes stable.
Beta-decay occurs among the neutron-rich fission byproducts produced in nuclear reactors.
So here we could understand that the atomic nucleus will be unstable and have an excess of neutrons. It will undergo ${\beta ^ - }$ , where a neutron is converted into a proton, an electron, and an electron antineutrino. Neutrons are present in the nucleus of an atom.
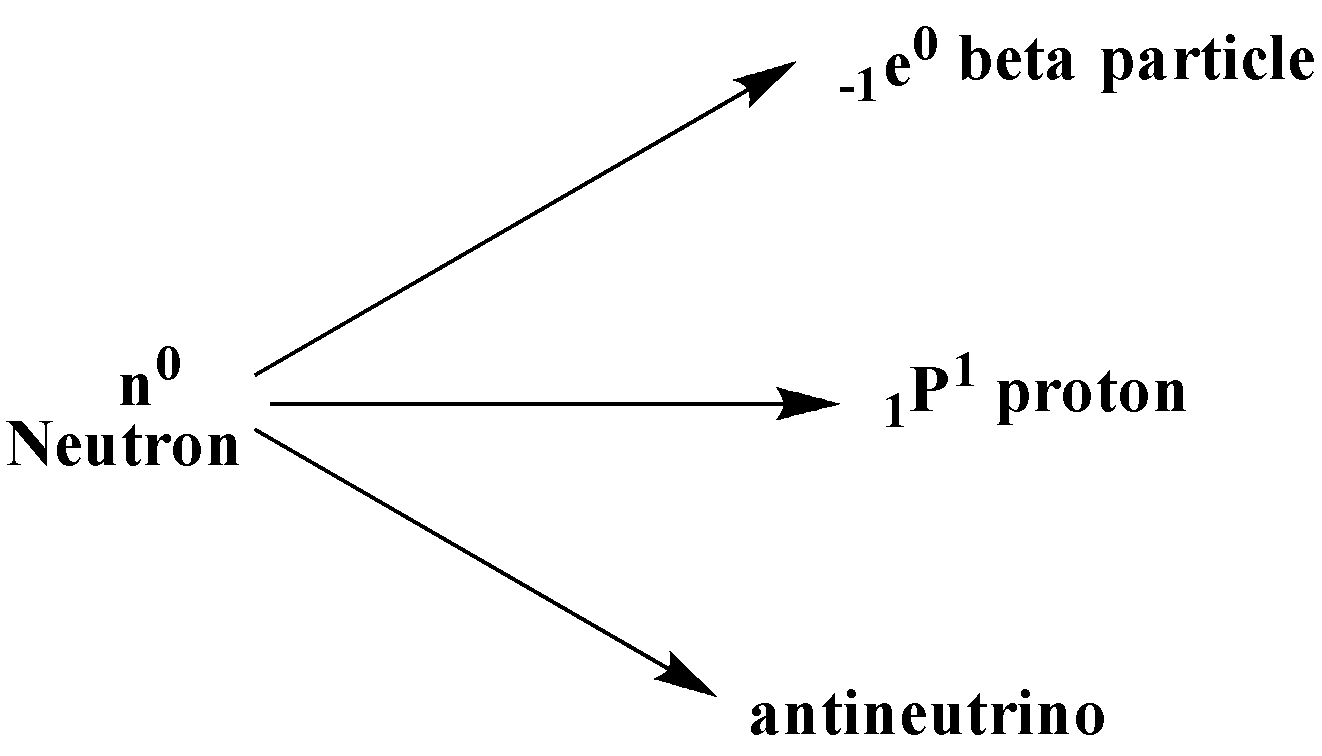
In ${\beta ^ + }$ decay, the unstable atomic nuclei have an excess of protons undergo a positron decay, where the proton is converted into a neutron, a positron, and an electron. A proton is also present inside the nucleus of an atom.
Thus, we can say that in a beta emission, the electrons are ejected from the nucleus of an atom since neutrons are present in the nucleus of an atom.
The correct answer is an option (D).
Note:
Strontium-90 is used to produce beta particles. Beta particles are also used to treat health conditions such as eye and bone cancers and are also used as tracers. The beta particle can be stopped by an aluminum sheet or a few meters of air. It is used in quality control to check the thickness of some items.
Complete step by step answer:
As we know that a beta particle is also called beta ray or radiation. It is a high energy and high-speed electron or positron that is emitted by the radioactive decay of the atomic nucleus.
There are 2 types of beta decay, one in which electrons are produced and are ${\beta ^ - }$ decay, and one in which positrons are emitted is ${\beta ^ + }$ decay.
The radioactive atom has an unstable nucleus, hence when radiations are emitted, it becomes stable.
Beta-decay occurs among the neutron-rich fission byproducts produced in nuclear reactors.
So here we could understand that the atomic nucleus will be unstable and have an excess of neutrons. It will undergo ${\beta ^ - }$ , where a neutron is converted into a proton, an electron, and an electron antineutrino. Neutrons are present in the nucleus of an atom.

In ${\beta ^ + }$ decay, the unstable atomic nuclei have an excess of protons undergo a positron decay, where the proton is converted into a neutron, a positron, and an electron. A proton is also present inside the nucleus of an atom.
Thus, we can say that in a beta emission, the electrons are ejected from the nucleus of an atom since neutrons are present in the nucleus of an atom.
The correct answer is an option (D).
Note:
Strontium-90 is used to produce beta particles. Beta particles are also used to treat health conditions such as eye and bone cancers and are also used as tracers. The beta particle can be stopped by an aluminum sheet or a few meters of air. It is used in quality control to check the thickness of some items.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




