
In ${B_2}{H_6}$
A.There is direct boron-boron bond
B.The boron atoms are linked to the hydrogen bridges
C.The structure is similar to ${C_2}{H_6}$
D.All the atoms are in one place
Answer
558.6k+ views
Hint: We have to remember that the diborane is a chemical compound which exists in three different forms – diborane (2), diborane (4) and diborane (6). Here, in this particular question we will be discussing diborane (6). We have to remember that the diborane consists of two boron atoms bonded with six hydrogen atoms. The chemical formula is represented as ${B_2}{H_6}$.
Complete step by step answer:
We can draw the chemical structure of diborane as,
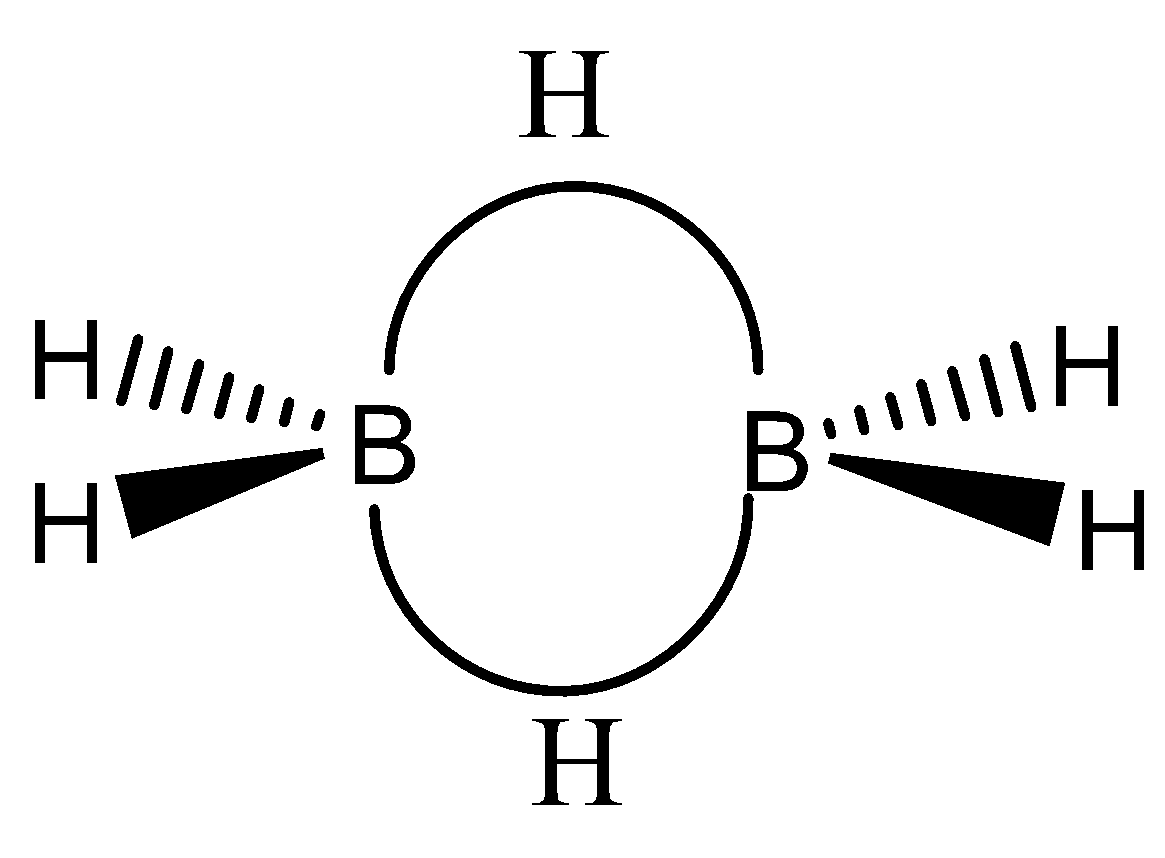
We can see in this structure there are two boron atoms linked to six hydrogen atoms, but there is no boron-boron bond. If we observe each boron atom, it is linked to two hydrogen atoms forming a strong bond and the two boron atoms are linked to each other with hydrogen bond bridges. If we look at the atoms, the atoms and its electrons are not all in the same place. In the hydrogen bridges one electron is shared in covalency.
We have to remember that the bond between the boron and hydrogen bridges is a three center sharing two electrons. This bonding is different from other molecules such as hydrocarbons.
If we consider ethane - ${C_2}{H_6}$ . The chemical structure can be observed as,
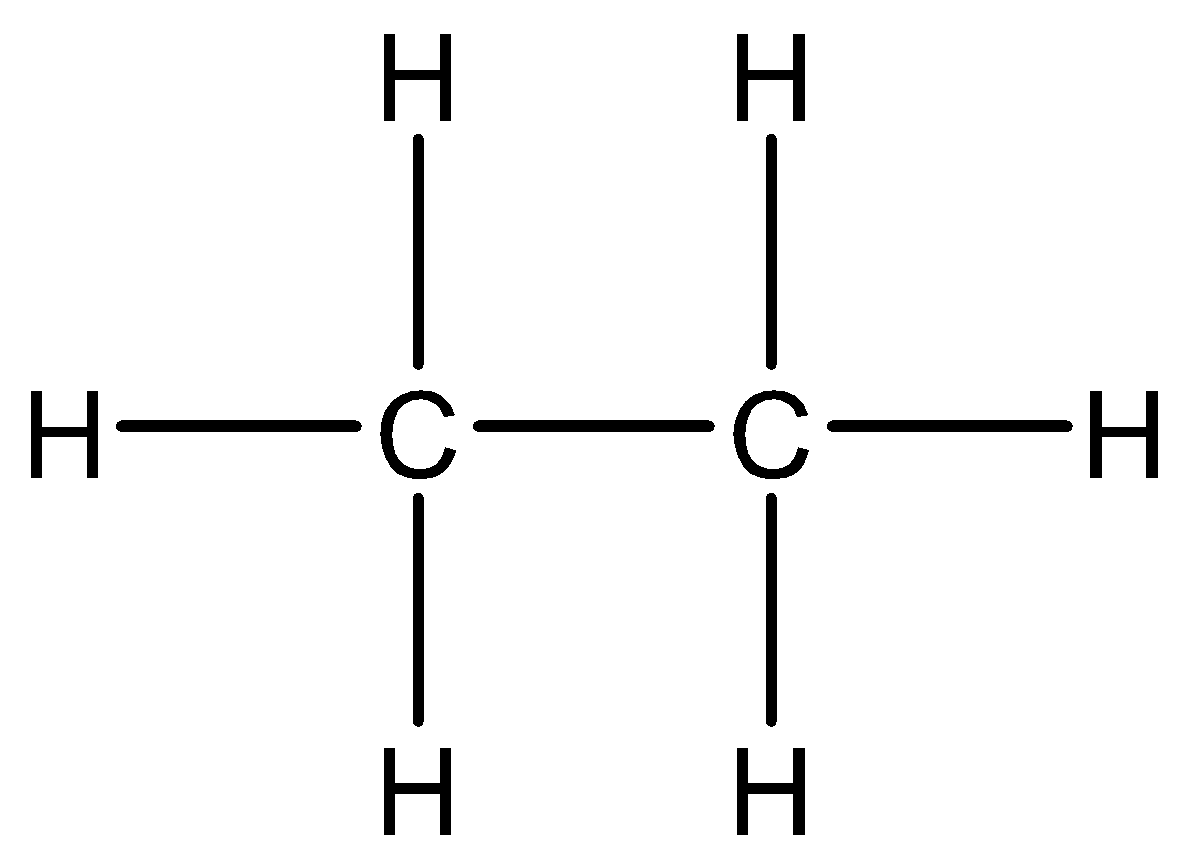
In this structure there is a carbon-carbon bond, which is not observed in case of diborane as there is no boron-boron bond. Hence, the structure is not similar.
From this study, we can observe that in diborane, the boron atoms are linked to hydrogen bridges.
Hence, the correct answer is option B.
Note:
We have to remember that the diborane is a colorless pyrophoric gas with a sweet odor. It is a highly reactive and versatile reagent. Due to its distinct property it is widely used in many applications. When it reacts with oxygen it can act as a propellant. In its application, diborane has been tested as a rocket propellant. The combustion is strongly exothermic. Diborane is also used as a rubber vulcanizer, also as a catalyst for hydrocarbon polymerization, as a flame-speed accelerator, etc.
Complete step by step answer:
We can draw the chemical structure of diborane as,

We can see in this structure there are two boron atoms linked to six hydrogen atoms, but there is no boron-boron bond. If we observe each boron atom, it is linked to two hydrogen atoms forming a strong bond and the two boron atoms are linked to each other with hydrogen bond bridges. If we look at the atoms, the atoms and its electrons are not all in the same place. In the hydrogen bridges one electron is shared in covalency.
We have to remember that the bond between the boron and hydrogen bridges is a three center sharing two electrons. This bonding is different from other molecules such as hydrocarbons.
If we consider ethane - ${C_2}{H_6}$ . The chemical structure can be observed as,

In this structure there is a carbon-carbon bond, which is not observed in case of diborane as there is no boron-boron bond. Hence, the structure is not similar.
From this study, we can observe that in diborane, the boron atoms are linked to hydrogen bridges.
Hence, the correct answer is option B.
Note:
We have to remember that the diborane is a colorless pyrophoric gas with a sweet odor. It is a highly reactive and versatile reagent. Due to its distinct property it is widely used in many applications. When it reacts with oxygen it can act as a propellant. In its application, diborane has been tested as a rocket propellant. The combustion is strongly exothermic. Diborane is also used as a rubber vulcanizer, also as a catalyst for hydrocarbon polymerization, as a flame-speed accelerator, etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




