
In Arrhenius plot, intercept is equal to:
A. $\dfrac{{{{\text{E}}_{\text{a}}}}}{{\text{R}}}$
B. ln A
C. ln K
D. ${\text{lo}}{{\text{g}}_{{\text{10}}}}{\text{a}}$
Answer
585.6k+ views
Hint: Arrhenius equation relates to the dependence of reaction rates on the temperature. The expression used to derive the intercept of the Arrhenius plot is ${\text{k = A}}{{\text{e}}^{{\text{ - }}{{\text{E}}_{\text{a}}}{\text{/RT}}}}$. This expression is also known as Arrhenius equation. The plot is drawn in between the values of ln K and 1/T.
Complete step by step answer:
First, let us know about the Arrhenius equation. As mentioned above the equation can be written as:
${\text{k = A}}{{\text{e}}^{{\text{ - }}{{\text{E}}_{\text{a}}}{\text{/RT}}}}$
Now, as we know that in the Arrhenius equation k depicts the rate constant of the reaction. A is considered to be the pre-exponential factor, T is temperature, R is the universal gas constant, and is the activation energy used in the chemical reaction.
Here, we have to find the intercept of the equation. So, let us consider logarithm on both sides, then it can be written as:
${\text{lnK = ln(A}}{{\text{e}}^{{\text{ - }}{{\text{E}}_{\text{a}}}{\text{/RT}}}}{\text{)}}$
If we solve it further then it can be written as:
${\text{lnK = lnA - }}\dfrac{{{{\text{E}}_{\text{a}}}}}{{{\text{RT}}}}$
Now, we will compare this equation with another equation, i.e. y = mx + c
In this equation m represents the slope, and c represents the intercept of the plot.
Thus, after the comparison of the equations, we get slope = -$\dfrac{{{{\text{E}}_{\text{a}}}}}{{\text{R}}}$, and the intercept is equal to ln A.
We can see the plot of the dependence of reaction rate on the temperature which indicates the Arrhenius plot as shown:
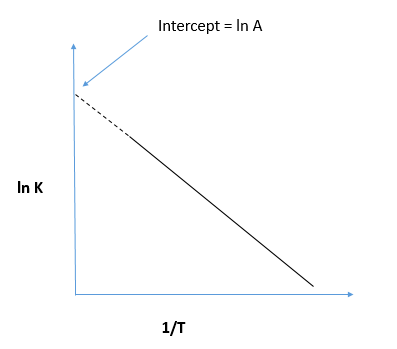
In the last we can conclude that in the Arrhenius plot, the intercept is equal to ln A.
Hence, the correct option is B.
Note: In this question, we have compared the Arrhenius equation with the equation representing straight lines. As mentioned, the graph between ln k, and 1/T is a straight line. According to the mathematical concept, the straight line equation depicts the slope, and intercept of the plot.
Complete step by step answer:
First, let us know about the Arrhenius equation. As mentioned above the equation can be written as:
${\text{k = A}}{{\text{e}}^{{\text{ - }}{{\text{E}}_{\text{a}}}{\text{/RT}}}}$
Now, as we know that in the Arrhenius equation k depicts the rate constant of the reaction. A is considered to be the pre-exponential factor, T is temperature, R is the universal gas constant, and is the activation energy used in the chemical reaction.
Here, we have to find the intercept of the equation. So, let us consider logarithm on both sides, then it can be written as:
${\text{lnK = ln(A}}{{\text{e}}^{{\text{ - }}{{\text{E}}_{\text{a}}}{\text{/RT}}}}{\text{)}}$
If we solve it further then it can be written as:
${\text{lnK = lnA - }}\dfrac{{{{\text{E}}_{\text{a}}}}}{{{\text{RT}}}}$
Now, we will compare this equation with another equation, i.e. y = mx + c
In this equation m represents the slope, and c represents the intercept of the plot.
Thus, after the comparison of the equations, we get slope = -$\dfrac{{{{\text{E}}_{\text{a}}}}}{{\text{R}}}$, and the intercept is equal to ln A.
We can see the plot of the dependence of reaction rate on the temperature which indicates the Arrhenius plot as shown:

In the last we can conclude that in the Arrhenius plot, the intercept is equal to ln A.
Hence, the correct option is B.
Note: In this question, we have compared the Arrhenius equation with the equation representing straight lines. As mentioned, the graph between ln k, and 1/T is a straight line. According to the mathematical concept, the straight line equation depicts the slope, and intercept of the plot.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




